Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2013) Volume 1, Issue 2
Biomass is one of the main renewable energy sources and coupled with carbon dioxide adsorbent material, such as calcium oxide sorbent, it increases the biomass conversion efficiency during gasification. This study aims to investigate the thermal degradation behavior of biomass and biomass/sorbent blends. Thermal stability is the stability of the material to resist change in physical shape as its temperature change. The purpose of this study is to determine the thermal stability and the conversion efficiency of biomass and biomass with sorbent blends. Thermo gravimetric analysis (TGA) was employed to determine the thermal stability of biomass and sorbent mixtures of pine-wood, calcium oxide (CaO), and or magnesium oxide (MgO), which will ultimately determine the gasification characteristics of the blends. A mixture resulted in the highest thermal stability and conversion efficiency compared to others will be the one suitable for gasification.
Keywords: Thermal analysis; Biomass; Thermal degradation
Renewable energy is of growing importance in satisfying environmental concerns over fossil fuel usage. Wood and other forms of biomass, including energy crops and agricultural and forestry wastes, are some of the main renewable energy resources available [1]. It has been observed that the combustion of fossil fuels has negative effect on the global climate world-wide. Relying on fossil fuels as the main energy sources has led to the serious energy crisis and environmental problems, such as fossil fuel depletion and pollutant emission [2]. Biomass is recognized as one of the major sources of renewable energy [1]. Biomass gasification involves heating of the biomass with controlled amount of air, oxygen, steam or various mixtures of these to produce gases, such as hydrogen (H2), carbon monoxide (CO) and methane (CH4) [3]. These gases are known as producer gas or synthesis gas (syngas). Gasification can use low-value feedstocks and convert them not only into electricity, but also into transportation fuels [4]. Depending on the gasification agent used, syngas could contain a large quantity of nitrogen, carbon dioxide and traces of water.
It is very important to know the thermal behavior of biomass and biomass/sorbent blends for the purpose of development of thermo chemical conversion systems of the material. Lignin-cellulosic materials are more reactive and have higher volatile matter content than coals. Therefore, its fundamental characterization is required, which exhibit very different properties with respect to traditional fossil fuels. The behavior of biomass materials during gasification has often been referred to the behavior of chemical components (cellulose, hemicellulose, and lignin) [5,6]. Thermo gravimetric analysis (TGA) is a method which can be used to study the thermal behavior of carbonaceous material [7]. This method provides important information on the devolatilization of materials, that is, the identification of major volatile species and the typical temperature range of release, with a continuous measurement [8].
The mineral matter present in the fuels influences devolatilization behavior, and this may cause some differences in the behavior of natural biomasses and synthetic fuels (obtained as a blend of cellulose, hemicellulose, and lignin) [9]. Although a considerable number of studies have been carried out to evaluate the effect of mineral matter on biomass pyrolysis, a detailed understanding has not been obtained yet [10]. This paper aims at studying the change in sample composition and thermal behavior of biomass and biomass with sorbent blends using TGA at different heating rates, and to determine the total degradation rate and the residual weight, for gasification purposes. The program developed by Chen [11] and modified by Jayah et al. [12] was used was used to predict the conversion efficiency achieved during the gasification of biomass with sorbents, as compared to the gasification of biomass alone. The material which is more thermally stable and has higher efficiency compared to others will be used for gasification.
Six samples representing various compositions of biomass (pinewood) with calcium oxide (CaO) and magnesium oxide (MgO) sorbents were analyzed by Thermo gravimetric Analysis (TGA). These mixtures were compared with the pure wood material. Pine wood was coarse ground to 250 µm, so that it can homogeneously mixtured with sorbents material. CaO and MgO powders were used without any further preparation. The experiments were carried out in a nitrogen atmosphere with the ga flow rate maintained at 20 ml/min at different heating rates of 10, 15 and 20°C/min in a temperature of 20°C to 900°C. The ratio of the mixtures of biomass and sorbents is presented in Table 1. Proximate analysis (moisture content, volatile matter, fixed carbon and ash) was determined from the TGA graph. Ultimate analysis was determined using carbon, hydrogen, nitrogen and sulfur (CHNS) analyzer. Proximate and ultimate results are presented in Table 2. Proximate and ultimate results were used as imput parameters in a downdraft gasifier simulation program developed by Chen [11] and modified by Jayah et al. [12]. The progam determines the mass and energy balance as well as the conversion efficiency.
| Method | Wood (%) | CaO (%) | MgO (%) | CaO.MgO (%) | Sample |
| 1. | 100 | - | - | - | Wood |
| 2. | 90 | 5 | 5 | 10 | 10% CaO.MgO |
| 3. | 85 | 7.5 | 7.5 | 15 | 15% CaO.MgO |
| 4. | 80 | 20 | - | - | 20% CaO |
| 5. | 80 | - | 20 | - | 20% MgO |
| 6. | 75 | 12.5 | 12.5 | 25 | 25% CaO.MgO |
Table 1: The mixture of pine wood, CaO and MgO materials.
| Proximate analysis | Ultimate analysis | |||||||
| Sample | Moisture content | Fixed carbon | Volatile matter | Ash content | Carbon | Hydrogen | Nitrogen | Oxygen |
| Pine wood | 8.6 | 23.8 | 67.72 | 0.40 | 47.51 | 6.52 | 0.09 | 45.87 |
| 10% Cao.MgO | 6.0 | 29.86 | 59.28 | 4.86 | 43.24 | 6.28 | 0.08 | 50.40 |
| 15% CaO.MgO | 6.0 | 32.24 | 47.10 | 4.66 | 43.19 | 6.23 | 0.068 | 50.46 |
| 20% CaO | 5.6 | 22.48 | 56.42 | 15.7 | 39.33 | 5.99 | 0.85 | 54.59 |
| 20% MgO | 5.9 | 21.3 | 58.37 | 13.9 | 40.16 | 5.47 | 0.82 | 54.29 |
| 25% CaO.MgO | 5.0 | 26.32 | 50.42 | 18.26 | 45.37 | 6.50 | 0.07 | 48.07 |
Table 2: Proximate and ultimate analysis of biomass and biomass/sorbents blends.
Simulation program
The computer software was basically a model developed for the downdraft wood gasifiers to study the effects of operating and design parameters on reactor performance [11]. It consists of two submodels, namely flaming pyrolysis and gasification zone sub-models. Flaming pyrolysis zone sub model is used to determine the product concentration and temperature of gas leaving the flaming pyrolysis zone. The gasification zone sub-model is used to predict the output of the product gas and the length of the gasification zone at any given time step [13]. The principle of mass and energy balance was also used.
Flaming pyrolysis zone sub model: In the flaming pyrolysis zone, the general equation of reaction of wood can be expressed by Equation 1:
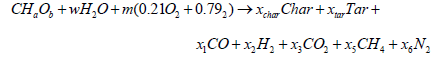 (1)
(1)
Here, char was taken as carbon and ultimate analysis of tar as CH1.03O0.03 [14]. From Equation 2 and 3, we can obtain the equilibrium equation and the corresponding equilibrium constant, respectively.
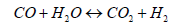 (2)
(2)
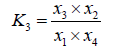 (3)
(3)
The correlation between the temperature and equilibrium constants for the above is given by Equation 4 [15].
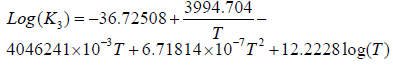 (4)
(4)
Where T is the temperature (K)
By mass balance, the following Equations 5-8 can be obtained:
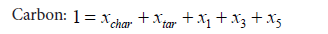 (5)
(5)
Hydrogen: 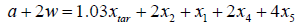 (6)
(6)
Oxygen: 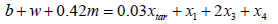 (7)
(7)
Nitrogen: 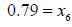 (8)
(8)
The energy balance in flaming pyrolysis zone is given by 9:
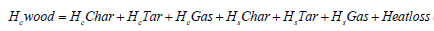 (9)
(9)
w is the number of moles of water, including fuel moisture, air moisture and water or steam addition [11]. It can be calculated by the following equation.
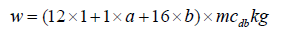
Moisture in fuel wood=dry matter in fuel wood×moisture content on dry basis
a and b is given, heat loss and m (number of moles of oxygen input) are obtained from the experiment, x5, char and star are assumed, x1, x2, x3, x4, x6 and T are solved by using the successive approximation method with a Fortran program. The higher heating value (kJ/g) of wood, char and tar are calculated from the equation as follows [16].
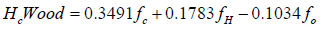
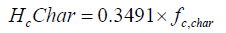
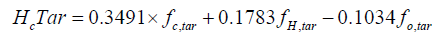
The chemical energy content of output gas and sensible energy of char, tar and output gases are calculated as follows:
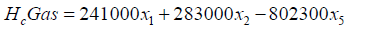
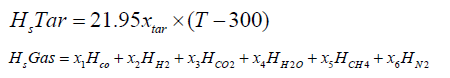
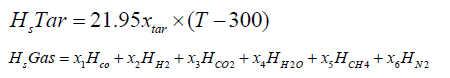
Gasification zone sub-model: The gasification zone is modelled by following a particle along the axis of the reactor. The computer program has been formulated using FORTRAN language to calculate the characteristic profiles along the reactor axis. The profile includes temperature, concentrations, efficiency and distance the particle travelled. The length co-ordinate is coupled with a time variable through the solid phase velocity. A small time increment approach is used in calculating the product composition of the zone. It involves the use of a step procedure starting from the gasification zone and marches axially through the reactor in appropriate time increments. The output values of the flaming pyrolysis zone are used as inputs for modelling the gasification zone [13].
Conversion efficiency
Gasification efficiency is the percentage energy of biomass converted into a cold producer gas (free from tar). The average energy conversion efficiency of wood gasifiers is about 60-70% [17], and is defined as:

The Figures 1-3 show the TGA (left hand side) and DTG (right hand side) curves of wood, wood with 10% CaO.MgO, 15% CaO.MgO, 20% CaO, 20% MgO and 25% CaO.MgO mixtures at the heating rate of 10, 15 and 20°C/min, respectively. The descending TGA thermal curve indicates that a weight loss has occurred. From both curves, the initial thermal degradation temperatures are due to weight loss of water vaporization up to 120°C, followed by devolatilization, which is the major step in all thermochemical conversion processes involving biomass. This step is represented by the second stage of decomposition, occurring at temperature between 200 to 450°C in the case of wood, and between 200 to 580°C for the mixtures. This is where the remarkable slope of the TGA curves is observed. The main decomposition of wood region is shown by decomposition regions of hemicellulose, cellulose and lignin. The weight loss of hemicellulose happens in the region around 220-315°C and cellulose around 315-450°C, with maximum weight loss at 323°C [18]. This maximum weight loss is confirmed by DTG graphs. Lignin decomposition is in the range from 200°C up to 900°C, with an unclear maximum weight loss. This implies that lignin is decomposed in both stages of hemicellulose and cellulose decomposition. The small decomposition peak appears at temperatures 540°C and 694°C, in the case of wood with 20% CaO and 25% CaO. MgO mixtures and it can be attributed to the carbonation of CaCO3, through which CaO can absorb the released CO2 to form CaCO3 product. The calcium peaks are mainly in the form of hydroxides and carbonates, which during heating in the TGA apparatus decompose releasing H2O and CO2 [8].
The biomass decomposes faster that biomass/sorbent blends at lower temperatures. This implies that the biomass/sorbent mixtures can withstand higher temperatures without degrading at a fast rate. From Figure 1 and 2, it can be observed that the mixture of wood with 20% CaO is the most thermally stable compared to other samples. It was found that there was up to 90% difference between the rate of degradation of wood and the blend of 20% CaO,whereas Figure 3 shows that the 25% CaO.MgO sample is more thermally stable than other samples. The sample weight of wood seemed to be more constant after the temperature reach 460°C. However, the samples with sorbents are constant after 500°C. The trend is due to the high volatile content in biomass as compared to biomass/sorbent blends. This implies that the addition of oxides to biomass decreases the volatile content of the blends, as evident in Table 2.
In both the graphs, the last stage in the case of wood and the blends is associated with the remaining ashes and the carbonates after gasification, respectively. Very low quantity of ash is observed for wood sample (0.5%). However, the sorbents have between 5 and 20% of the remaining carbonates. This is due to high volatility maters (70-80%) in biomass.
The thermal analysis of the samples used in this study shows higher thermal stability of wood with 20% CaO and 25% CaO.MgO samples. The TGA results and the gasification simulation results were used to select the sample with high thermal stability and enhanced conversion efficiency, respectively. The high thermal stability implies that the material will release energy over extended periods, while high conversion efficiency implied that more energy will be converted, and will be available or end use.
Figure 4 shows the conversion efficiency of of wood, wood with 10% CaO.MgO, 15% CaO.MgO, 20% CaO, and 20% MgO 25% CaO.MgO samples as a function of time. This was obtained using the computer program discussed in section 2.1. The proximate and ultimate results together with the gasification parameters (Table 3) were used as input parameters for the determination of the conversion efficiency.
| Parameters | Values |
| Bulk density (g/cm3) | 0.4 |
| Diameter of wood particle (cm) | 2.5 |
| Throat diameter (cm) | 10 |
| Throat angle (degrees) | 30 |
| Insulation thickness (cm) | 17.5 |
| Thermal conductivity (w/cm.K) | 2.8 |
| Temperature of air input (K) | 900 |
| Feed input (kg/hr) | 50 |
| Air input (kg/hr) | 44.5 |
| Heat loss (%) | 12.8 |
Table 3: Gasification simulation parameters.
It can be observed from this figure that wood with 25% CaO. MgO mixture resulted in higher efficiency (78%) compared to other mixtures. This was achieved with the throat diameter and angle of 10 cm and 30 degrees, respectively. Dolomite (CaO.MgO) is known for cracking tars in the gasifier at high temperature, which leads to high production of producer gas. Because the sorbent material in the gasifier captures carbon dioxide, thereby releases heat, which then increases the temperature, which results in an increase in cracking of tar and conversion of the char present [19]. By so doing, it means producer gas in the gasifier is increased, hence an increase in conversion efficiency.
Figure 5 shows the percentage difference between wood and wood with 25% CaO.MgO blend. A significant difference (20%) was observed, implying that the sorbent is effective in enhancing the conversion efficiency of the gasification process.
This aim of this study was to determine the thermal stability and the conversion efficiency of biomass and biomass/sorbent blends using TGA and computer simulation, respectively. The high thermal stability was obtained with wood with 20% CaO at the heating rates of 10°C/ min and 15°C/min, whereas at the heating rate of 20°C/min wood with 25% CaO.MgO was more stable compared to others. The simulations of these samples were conducted and the highest conversion efficiency (78%) was obtained from wood/25% CaO.MgO mixture.