Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2013) Volume 3, Issue 2
In this work anew wavevector analysis of Elastic Incoherent Neutron Scattering (EINS) data on bioprotectant systems based on a wavelet approach is presented. The wavevector analysis allows to compare the spatial properties of the three systems such as three glass-forming homologous disaccharides (trehalose, maltose and sucrose),in the wavevector range of Q=0.28-4.27 Ǻ-1 and the wavelet transform reveals the existence of different kinds of protons dynamics. The comparison among the EINS spectra of the investigated systems points out systematically lower and sharper contributions for trehalose than for maltose and sucrose both at low and high wavevector, so highlighting a less extended global energy distribution along the wavevector range for trehalose.
<Keywords: Elastic incoherent neutron scattering; Wavelet analysis; Disaccharides; Thermal properties
Over recent years a huge effort has been dedicated to the elucidation of the mechanisms responsible for the capability of many organisms to survive under environmental stress conditions [1-10]. Homologous disaccharides (C12H22O11), e.g. sucrose, maltose and trehalose,play an important role in nature as bioprotectant systems. In particular, trehalose(α-D-glucopyranosyl, α-D-glucopyranoside), a non-reducing sugar, is synthesized by several organisms, called “extremophiles” [11-18] to overcome harsh conditions by entering into a state of suspended animation, called “cryptobiosis”. To exemplify, trehalose is one of the survival strategies for tardigrada, microorganisms which are classified as “polyextremophiles” due to their capability to survive at very low and very high temperatures, hard vacuum and high radiation concentrations; Artemia Salina, a crustacean surviving at high salinity levels, and Resurrection plants, living several years in dry conditions.
Although many studies have been focused on ternary systems such as biostructure/water/disaccharide [19-27], many researchers retain that the protein dynamics is strongly coupled with, and depends on, the solvent properties [28-36] and, for this reason, their attention has been mainly addressed to the disaccharide/water mixtures. Many light and neutron scattering findings on disaccharide/water mixtures indicate that the molecular mechanisms underlying the trehalose bioprotective effectiveness lie on the peculiar interaction between trehalose and water [37-41]. Green and Angell [42] have hypothesized that the bioprotectant effectiveness of trehalose could be related to the higher value of its glass transition in comparison with its homologous. However, other systems, such as dextran, present a comparable Tgvalue, but do not show an analogous bioprotective action. On the other hand, Crowe et al. [43] suggests a direct interaction between the sugar and the biomolecule : in particular their “water replacement hypothesis” justifies the trehalose protective function with the existence of direct hydrogen bonding of trehalose with the polar head groups of the lipids. This hypothesis was strengthened by the simulation reported by Donnamaria et al. [44], which argue that the structure of trehalose is perfectly adaptable to the tetrahedral coordination of pure water, whose structural and dynamical properties are not significantly affected by trehalose.
As a matter of fact experimental findings obtained by several spectroscopic techniques indicate clearly that the structural and dynamical properties of water, even at relatively low sugar concentration, result drastically perturbed by disaccharides, and in particular by trehalose [45-59].
More specifically, neutron diffraction results [53] show for all disaccharides, and for trehalose to a larger extent, a significant perturbation of the spectral contributions associated with the water hydrogen-bonded network that can be attributed to the destroying of the tetrahedral coordination of pure water. Simulations by the EPSR code [54], performed in combination with further neutron diffraction investigations, confirm that the water structure is strongly perturbed by trehalose, with an effect on the water second hydration shell resembling that produced by high pressure. Coherently Raman spectroscopy, by the analysis of the intramolecular OH stretching vibration band, shows that the addition of trehalose, in respect to the other disaccharides, more deeply destroys the tetrahedral intermolecular network of water, which by lowering temperature would give rise to ice. As a confirmation, Uchida et al. [55], detecting freeze-fractured replica images of the three disaccharides by a field-emission type transmission electron microscope (FE-TEM), confirm that trehalose, in respect to the other disaccharides, has a higher inhibitory effect on the growth of ice crystals. QENS experiments [17,37,38] show that also the water dynamics is significantly affected by the presence of disaccharides and in particular by trehalose, while Lerbretet al. [56] by molecular dynamics simulation studies confirmed for trehalose a higher distortion of the hydrogen bonded network of water from its tetrahedrality and have shown that the relaxation times of water in the presence of disaccharides result 1.2 to 10 times longer than those of pure water.
Cordoneet al. [57] and Cottoneet al. [58] by complementary techniques showed that sugar matrices lock the surface of the protein hindering large amplitude solvent coupled protein motions.
Finally, Caliskan et al. [59] have investigated the influence of glycerol and trehalose on lysozyme by Raman scattering showing that protein dynamics couples to that of trehalose and glycerol with glycerol providing a higher suppression of protein dynamics than trehalose at low temperatures, while trehalose is more effective than glycerol at higher temperatures.
It is well known that neutrons with a 1Å wavelength and an energy close to 1 kcal/mol represent an excellent probe to characterize thermal molecular motions and conformational changes in biological systems [60-67]; this is essentially due to the time-space scale to which is sensitive, to the simplification brought about by the neutron-nucleus interaction and to the distinctive isotopic character. From the experimental point of view, the characterization of the different molecular processes involved in the dynamics of some systems of biophysical interest can be successfully investigated by Elastic Incoherent Neutron Scattering (EINS) [68] by means of the so called “fixed-windows” method [69], where the scattered intensity is collected at ω=0 with a fixed “energy windows” corresponding to the instrumental energy resolution. As far as the wavelet analysis is concerned, it is possible to extract information from a non-stationary signal in terms of functional forms called “mother wavelet”. More precisely, the translated-version wavelets locate where one concerns, whereas the scaled-version wavelets allows to analyze the signal in different scale [70-72]. The square of the modulus of the wavelet transform is called scalogram, which transform shows how the energy of the signal varies as a function of the independent variable and of its conjugate variable.
In a previous work [73] a wavelet analysis has been performed on EINS data in order to localize anomalies in the trend of the Mean Square Displacement (MSD) against temperature; in the present work the aim is to report the findings of a new wavelet analysis of EINS intensity data on three homologous disaccharides water mixtures. This analysis allows to compare the spatial properties of the three systems revealing the existence of different kinds of protons dynamics in different wavevector ranges. In particular, it will be shown that the scalogram of the signal, i.e. of the elastically scattered intensity, along the wavevector range for trehalose is markedly less extended in respect to the other two disaccharides. In other terms, these findings point out a lower flexibility and a lower fragile character of the trehalose matrix in which biostructures are immersed, so highlighting the different nature of the involved dynamical processes in bioprotection that can justify the highest trehalose “cryptobiotic” effectiveness.
Ultrapure powdered sucrose, maltose and trehalose, and H2O, purchased by Aldrich-Chemie, were used to prepare solutions at a weight fraction corresponding to 6 and 19 water molecules for each disaccharide molecule. Measurements were performed in the temperature range of 20–310K on hydrogenated sucrose, maltose and trehalose in H2O at a weight fraction value of φ=0.5, corresponding to 19 water molecules for each disaccharide molecule. At such a concentration value different spectroscopic techniques indicate that the disaccharides in water solution are bonded to more than ≈22 water molecules at room temperature, this hydration number increasing by lowering temperature. In the used IN13 configuration the incident wavelength was 2.23 Å and the Q-range was 0.28-4.87Å-1. Raw data were corrected for cell scattering and detector response and normalized to unity at Q= Å-1. EINS measurements have been carried out across the glass transition temperature values on sucrose, maltose and trehalose/ H2O mixtures by using the IN13 backscattering spectrometer at the Institute Laue Langevin (ILL, Grenoble, France). The IN13 peculiarity is the relatively high energy of the incident neutrons (16 meV) which makes it possible to span a wide range of momentum transfer Q(≤4.87 Å-1) with a very good energy resolution (~8μeV).
With the aim of to clarify the reasons that make trehalose the most effective bioprotectant among the investigated homologous disaccharides, in the present work the attention is addressed to the differences in the dynamical behavior of the water mixtures of trehalose, maltose and sucrose. More specifically, a wavevector analysis of these EINS data through a wavelet approach is performed. Such an analysis puts into evidence, for the three investigated disaccharide mixtures by varying temperature, the existence of different kinds of protons dynamics which interest different wavevector ranges.In the present paper, the Mexican hat has been considered, which is defined:
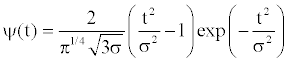
In Figure 1, as an example, the 3D scalograms as resulting from a wavelet analysis for trehalose/water mixtures, at the concentration value of 19 H2O for each disaccharide molecule, for three different temperature values, i.e. T=19 K (a), 264 K (b) and 284 K (c), are shown. The employment of the wavelet analysis to the whole set of experimental data puts into evidence the presence of two distinct kinds of motions which cover different space ranges. In particular, at the lowest investigated temperature, 19 K, only one broad spectral contribution which spans in the whole investigated wavevector range, i.e. from Q=0.27 Å-1 to Q=4.27 Å-1, is revealed, as reported in Figure 1 (a); such a spectral contribution can be assigned mainly to the vibration motions of the system protons. As reported in Figure 1 (b), which reports the 3D scalogram at the intermediate temperature value of T= 264 K, by rising temperature a different contribution, at low wavevector values and specifically below Q=0.25 Å-1,clearly emerges. Finally, at the temperature value of T=284K (c) the weight of the low Q contribution increases with temperature Figure 1 (c).
In order to better highlight the different behaviour in the investigated systems, figure 2 shows a comparison of the scalogram for sucrose, maltose and trehalose at the intermediate temperature of T=264 K. As it can be seen, the spectral energy density distribution as a function of the wavevector shows different features for the three disaccharides increasing temperature. In particular, the energy distribution along the wavevector range appears to be markedly less broadened for trehalose in respect to its homologous. More in detail, for the case of trehalose/water mixture the low Q contribution is less high and wide in respect to sucrose/water mixture. Furthermore the minor side contribution appears to be more pronounced and covers a lower wavevector range. Therefore the wavevector contribution for trehalose by increasing temperature is constantly sharper.
Such a scalogram comparison allows to highlight both the differences among the homologous disaccharides, with a specific reference to the different explored wavevector ranges, and a higher thermal restrain for trehalose in respect to the other homologous disaccharides.
The wavevector analysis performed by wavelet transform of EINS data allows to compare the spatial properties of trehalose, maltose and sucrose, in the wavevector range of Q=0.28-4.27 Å-1, revealing the existence of different kinds of protons dynamics. The wide explored wavevector range allows to characterize and to compare the system molecular motions according to their spatial extent and amplitude. The experimental results reveal that the scattered intensity shows an almost linear trend at the lowest temperature, T=19 K, whereas, at higher temperature values, it drops in Q fulfilling a decaying behaviour which results less marked in the case of trehalose in respect to sucrose and maltose.
By the wavelet analysis of the scattered intensity as a function of exchanged momentum, for the three disaccharide/H2O mixtures, the existence of two different classes of protons dynamics , which explore different wavevector ranges, is shown. More specifically, at the lowest temperature, 19 K, only one spectral contribution is revealed; such a wide and flat contribution spans the whole wavevector range and is almost equal for all the investigated disaccharide/water systems; it can be attributed to the vibrational motions of the scatterer particles, i.e. the protons. At higher temperature values, the weight of the low Q contribution tends to increase and shows a different increasing rate for the three homologous disaccharides. These findings confirm that the system spectral energy density as a function wavevector is distributed in a different way for the three disaccharides:for trehalose/water mixture the low Q contribution is less high and wide and the side contribution is more pronounced and covers a lower wavevector range.
What it emerges is that both the low and high wavevector contributions for trehalose, at all the investigated temperature values, are constantly lower and sharper, giving rise to a global energy distribution along the wavevector range markedly less extended. It is possible to conclude that the structural resistance to temperature changes and the system rigidity decrease by following the order trehalose â?¦ maltose â?¦ sucrose.
By a molecular point of view, the implications of the present EINS findings are strictly connected to the biological aspect. More specifically, they elucidate molecular mechanisms of the flexibility/ stability relation. Extreme conditions induce both structural and dynamical instability which affects the biomolecular functional states. If a constant equilibrium between molecular stability and structural flexibility is maintained, biomolecular and metabolic functions can be preserved even in harsh external conditions. In this frame, a key role is played by trehalose, which is capable to encapsulate biostructures in a more rigid and more temperature insensitive environment in respect to other two disaccharides. In this glassy shell, biomolecules are preserved maintaining the requested delicate balance between rigidity and molecular fluctuations and then their fundamental biological functions.