Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2018) Volume 8, Issue 1
The inhibitive action of the methanol extracts of three powerful eco-friendly green Inhibitors; Costus afer (COA), Uvaria chamae (UVC), and Xylopia aethiopica (XYA) leaves on the corrosion of mild steel in 2.5 M HCl solutions with inhibitor concentrations of 0.5 g/L, 1.0 g/L, 2.0 g/L and 4.0 g/L, at the temperatures of 30°C and 60°C was studied using gravimetric (weight loss) and gasometric (hydrogen evolution) techniques to determine their inhibition efficiencies as well as to characterize the mechanism of inhibition of these three green inhibitors. The gravimetric technique was done for 5 days (120 Hours). Results indicate that the leave extracts inhibit the corrosion process powerfully. COA, UVC and XYA extracts showed inhibition efficiency of (83.7, 84.6 and 87.0) and (85.0, 62.5 and 76.1) for gravimetric (weight loss) and gasometric analysis respectively. The Inhibition efficiency was found to increase with an increase in the extract concentration and decrease with an increase in time(days) and temperature. The inhibition efficiencies followed the trend XYA>UVC>COA and COA>XYA>UVC in gravimetric and gasometric analysis respectively. Thermodynamic considerations revealed that the activation energy, Ea increased in the presence of the plant extracts. The kinetic data confirmed the reaction process to be first order. Adsorption of the plant extracts on mild steel surface is an exothermic process and spontaneous as deduced by mostly negative Qads mean values of -7.40 KJ/mol, -2.14 KJ/mol and -32.18 KJ/mol for COA, UVC and XYA and negative ΔGads values of -9.28 KJ/mol and -12. 0 KJ/mol for COA, -9.46 KJ/mol and -11.23 KJ/mol for UVC and -7.73 KJ/mol and 6.29 KJ/mol for XYA at 30°C and 60°C respectively. The mechanism of adsorption proposed for the plant extract on the mild steel surface is physical adsorption. Experimental data obtained fit the Langmuir adsorption isotherm.
<Keywords: Corrosion inhibitors; Mild steel; Hydrochloric acid; Thermodynamic studies; Kinetic Studies; Uvaria chamae; Costus afer; Xylopia aethiopica
Industries depend heavily on the use of metals and alloys. The most challenging and difficult tasks for industries is the problem of corrosion in aggressive environment. Acid solutions are usually used to remove undesired scale of several industrial processes, and this usually leads to corrosion of the metal. The use of inhibitors is one of the most practical methods for protection against corrosion especially in acid solutions to prevent metal dissolution and acid consumption [1,2]. This can also reduce the corrosion rate [3]. Classical inhibitors of metal corrosion like Chromates, Phosphates, Molybdates, and synthetic organic inhibitors containing hetero-atoms such as Nitrogen, Oxygen and Sulphur have been extensively studied as corrosion inhibitors [4-6]. However, pure synthetic chemicals are expensive and most of them are toxic and not biodegradable. Concentration is now on the new class of environmentally favorable, renewable and inexpensive alternatives [7-12].
Plant extracts are non-toxic, biodegradable, eco-friendly and readily available and if successfully utilized, they have the potential to provide us with low-cost alternatives. This paper aims at broadening the application of Costus afer , Uvaria chamae and Xylopia aethiopica leaves extracts as corrosion inhibitors. Uvaria chamae is commonly known as bush banana or finger root. Its fruit is edible and widely eaten; it is also a medicinal plant used to treat fevers and has antibiotic properties [13]. Costus afer is an African specie of costus. It produces two flowers by bract and grows in moist places, swampy areas of transitional forest and rainforest. It is found to be medicinal in the treatment of snake bites and other infection [14]. Xylopia aethiopica is commonly known as Ethiopia, Negro pepper or pepper of guinea. The fruit has a slightly hooked cylindrical pod reaching 2-3 mm in width. The mature fruits which are green in color take a brown coloration on drying [15]. This research provides information on the possible use of the methanol extracts from UVC, COA and XYA leaves as sources of cheap, eco-friendly and non-toxic inhibitors, and to the best of our knowledge this is the first time the inhibitive properties of Uvaria chamae , Costus afer and Xylopia aethiopica are compared in a single paper.
Materials
The mild steel sheet was obtained from Uratta market, Aba in Abia State, Nigeria and has the composition as described by Okafor [7]. Each sheet is 0.08 cm in thickness and was mechanically cut into coupons of dimension 5.0 cm × 1.5 cm for both gravimetric and gasometric measurements, with a hole of uniform diameter to facilitate the suspension of the coupon in the test solutions. Before each measurement, the mild steel coupon to be used was polished with emery paper (sand paper) of variable grades (starting with the coarsest and gradually proceeding to the finest), degreased with methanol and dried in acetone. All chemicals used were of Analar grade and distilled water was used for their preparation.
Preparation of plant extracts
Costus afer , Uvaria chamae and Xylopia aethiopica leaves were collected from Amune Ovim, in Isuikwuato L.G.A of Abia State, Nigeria. The leaves were dried under room temperature away from direct sunlight until a constant weight was obtained after several days of drying. A mechanical grinder was used to turn the dried leaves into powder form. The powder was extracted with methanol in a soxhlet extractor. After recovering most of the methanol, the extracts were heated in a water bath until almost all the methanol has evaporated. Four (4) grams of each extract was digested in 1000 mL of 2.5 M HCl (assumed to be 4.0 g/L). The resultant solutions were kept for 24 hours, filtered and stored. From the stock solution, 0.5, 1.0, 2.0 and 4.0 g/L test solutions were prepared.
Gravimetric (weight loss) results
The weight loss of the mild steel was evaluated in grams as the difference in the initial and final weight of the coupons, and also the average of the weight loss was taken due to the triplicate immersion. The experiment was carried out for the Costus afer , Uvaria chamae and Xylopia aethiopica leaves extract using concentrations of 0.5 g/L, 1.0 g/L, 2.0 g/L, and 4.0 g/L at ambient temperature. As the coupons corrode, there is a reduction in the weight of the coupon and this reduction is directly related to the corrosion rate. From the weight loss data, the corrosion rates (CR) and inhibition efficiency were calculated using equation (1) and presented in Table 1 [8].
| Spice | Time (Hours) | Corrosion Rate (mg/cm2hr-1) | Inhibition Efficiency (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blank | 0.5 g/L | 1.0 g/L | 2.0 g/L | 4.0 g/L | 0.5 g/L | 1.0 g/L | 2.0 g/L | 4.0 g/L | ||
| COA | 24 | 7.27 | 3.33 | 2.49 | 2.99 | 1.45 | 54.2 | 65.7 | 68.5 | 80.1 |
| 48 | 10.08 | 8.94 | 5.71 | 3.22 | 1.77 | 11.3 | 43.3 | 62.4 | 82.4 | |
| 72 | 11.08 | 5.68 | 4.16 | 3.05 | 1.8 | 48.7 | 62.4 | 72.5 | 83.7 | |
| 96 | 12.31 | 7.79 | 6.03 | 3.84 | 2.39 | 36.7 | 51 | 68.8 | 80.6 | |
| 120 | 13.05 | 8.69 | 6.53 | 3.82 | 2.33 | 33.4 | 50 | 70.7 | 82.1 | |
| UVC | 24 | 16.83 | 14.55 | 10.6 | 10.6 | 7.69 | 44.9 | 59.8 | 59.8 | 70.9 |
| 48 | 14.65 | 13.82 | 13.3 | 9.66 | 7.38 | 53.2 | 54.9 | 67.2 | 75 | |
| 72 | 17.94 | 16.76 | 10.53 | 9.63 | 4.16 | 37.8 | 60.9 | 64.3 | 84.6 | |
| 96 | 18.55 | 17.98 | 12.78 | 12.36 | 8.31 | 30.4 | 50.5 | 52.1 | 67.8 | |
| 120 | 15.59 | 14.88 | 14.42 | 10.43 | 7.32 | 45.1 | 46.8 | 61.5 | 73 | |
| XYA | 24 | 0.7 | 0.42 | 0.57 | 0.51 | 0.54 | 33.3 | 56.8 | 69.1 | 75.3 |
| 48 | 0.47 | 0.32 | 0.23 | 0.14 | 0.1 | 23.7 | 46.4 | 66 | 75.3 | |
| 72 | 0.57 | 0.47 | 0.28 | 0.19 | 0.12 | 17.8 | 50.8 | 66 | 79.7 | |
| 96 | 0.51 | 0.48 | 0.25 | 0.19 | 0.08 | 6.7 | 50.6 | 62.9 | 83.5 | |
| 120 | 0.54 | 0.42 | 0.38 | 0.27 | 0.07 | 22.3 | 30.6 | 50.5 | 87 | |
Table 1: Weight loss data for mild steel coupon in Hydrochloric acid solutions containing different concentrations of extracts of Costus afer (COA), Uvaria chamae (UVC) and Xylopia aethiopica (XYA).
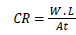 (1)
(1)
Where W.L. is weight loss in mg, A is the metal surface area and t, the time of immersion in hours. From corrosion rate, the surface coverage (θ) as a result of adsorption of inhibitor molecules and inhibition efficiencies of the plant extracts (I%) were determined using equation 2 and 3:
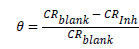 (2)
(2)
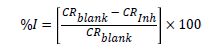 (3)
(3)
Where CRblank and CRinh are the corrosion rates in the absence and presence of the plant extracts respectively.
Hydrogen evolution results
The hydrogen evolution technique is based on the principle that corrosion reactions in aqueous media are characterized by the evolution of gas resulting from the cathodic reaction of the corrosion process. The rate of evolution of gas is proportional to the rate of corrosion [8,9]. The rate of evolution of the hydrogen gas (RH) is determined from the slope of the graph of volume of the hydrogen gas evolved (VH) versus time (t), and the inhibitors efficiencies (% I) were determined using equation (4):
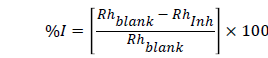 (4)
(4)
Where Rhblank and Rhinh are the corrosion rates in the absence and presence of the plant extracts respectively. The values for the rate of hydrogen evolution and inhibition efficiency are shown in Table 2. It was observed that the presence of the plant extracts decreased the rate of hydrogen evolution, and consequently decreased the corrosion rate of the mild steel in 2.5 M HCl solutions compared to the blank. The decrease is also dependent on the concentration of the plant extracts and temperature. Inspection of Table 2 shows that the rate of hydrogen evolution increased with increase in temperature and reduces with increase in concentration indicating that the plant extracts inhibit the corrosion of mild steel in 2.5 M HCl with increase in the concentration of the plant extracts. From the rate of hydrogen evolution, the inhibition efficiencies were determined. It was also observed that the inhibition efficiency increases with increase in the concentration of the plant extracts and decreased with increase in temperature indicating physical adsorption [9].
| Extract | Rate of Hydrogen Evolution (cm3/minute) | Inhibition Efficiency (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Blank | 0.5 g/L | 1.0 g/L | 2.0 g/L | 4.0 g/L | 0.5 g/L | 1.0 g/L | 2.0 g/L | 4.0 g/L | |
| 30°C | |||||||||
| COA | 0.08 | 0.06 | 0.03 | 0.03 | 0.1 | 21.3 | 65 | 66.3 | 85 |
| UVC | 0.11 | 0.08 | 0.07 | 0.06 | 0.04 | 27.7 | 38.4 | 48.2 | 62.5 |
| XYA | 0.09 | 0.07 | 0.06 | 0.05 | 0.02 | 18.2 | 33 | 38.6 | 76.1 |
| 60°C | |||||||||
| COA | 2.45 | 1.33 | 1.32 | 1.08 | 0.78 | 45.6 | 46 | 55.8 | 68.2 |
| UVC | 2.45 | 1.72 | 1.46 | 1.35 | 1.11 | 29.6 | 40.1 | 44.7 | 54.5 |
| XYA | 2.47 | 2.28 | 2.13 | 1.92 | 1.56 | 7.7 | 13.7 | 22.2 | 36.7 |
Table 2: Rate of Hydrogen evolution and inhibition efficiency for mild steel coupon in Hydrochloric acid solutions containing different concentrations of COA, UVC and XYA extracts.
Thermodynamic and adsorption considerations
Adsorption of inhibitor on metal surface is usually described in terms of adsorption isotherms. Thermodynamic adsorption isotherms provide information about the interaction among the adsorbed molecules themselves and also their interaction with the electrode surface [16]. Adsorption isotherms give a relationship between inhibitor concentration and the degree of surface coverage, and they are very important in understanding the mechanism of inhibition of corrosion species on the metal surface. Figures 1 and 2 show the Langmuir isotherms from the hydrogen evolution data at 30°C and 60°C, with correlation coefficients (R2) of (0.861, 0.992, 0.938) and (0.987, 0.997, 0.994) for COA, UVC and XYA respectively.
The Langmuir isotherm can be defined as:
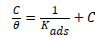 (5)
(5)
Where θ is the surface coverage, C is the concentration and Kads is the equilibrium constant of the adsorption process. From the intercept of the plots, Kads values have been obtained in Table 3 and related to the free energy of adsorption, ΔGads.
| Spice | Inhibitor Concentration (g/L) | Kads | ∆Gads (KJ/mol) | ∆Sads (KJ/mol) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ea (KJ/mol) | Qads (KJ/mol) | 30°C | 60°C | 30°C | 60°C | 30°C | 60°C | ||
| COA | Blank | 95.64 | - | 0.72 | 1.43 | -9.28 | -12 | 0.006 | 0.014 |
| 0.5 | 85.32 | 31.66 | |||||||
| 1 | 107.76 | -21.78 | |||||||
| 2 | 103.17 | -12.31 | |||||||
| 4 | 116.64 | -27.15 | |||||||
| UVC | Blank | 86.33 | - | 0.77 | 1.04 | -9.46 | -11.23 | 0.024 | 0.027 |
| 0.5 | 85.58 | 2.59 | |||||||
| 1 | 85.53 | 2.03 | |||||||
| 2 | 88.16 | -3.95 | |||||||
| 4 | 91.73 | -9.22 | |||||||
| XYA | Blank | 93.23 | - | 0.39 | 0.17 | -7.73 | -6.29 | -0.081 | -0.078 |
| 0.5 | 96.58 | -27.23 | |||||||
| 1 | 100.29 | -31.61 | |||||||
| 2 | 99.87 | -22.18 | |||||||
| 4 | 120.52 | -47.710. | |||||||
Table 3: Calculated Values of thermodynamic and adsorption parameters for COA, UVC and XYA from hydrogen evolution.
The Gibbs free energy was calculated using equation 6:
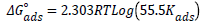 (6)
(6)
Where R is the molar gas constant (8.314 KJ/mol), T is the temperature in Kelvin and 55.5 is the molar concentration of water at the electrode-electrolyte interface [16]. The values of activation energy for the corrosion reaction in the presence and absence of the different extracts studied were calculated using condensed Arrhenius equation as follows:
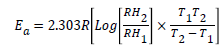 (7)
(7)
Where Ea is the activation energy of the reaction, R is the molar gas constant and RH1 and RH2 are the corrosion rate at T1 (30°C) and T2 (60°C), respectively. An estimate of the heat of adsorption (Qads) was obtained from the trend of surface coverage (θ) (% I=100 × θ) with temperature as follows;
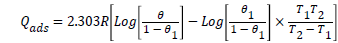 (8)
(8)
As the reactions were carried out at constant pressures, the values of Qads approximate those of the enthalpy of adsorption (ΔHads) [17]. Kads, ΔGads and ΔSads values were calculated from equations 6 and 9. It can be seen from the Table 3 that the values of equilibrium constant, Kads are positive, indicating better adsorption leading to high inhibition efficiency [18], and the ΔGads values obtained were almost negative, suggesting that the adsorption processes were spontaneous.
The entropy of adsorption, (ΔSads) was calculated using equation 9:
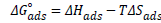 (9)
(9)
However, the Kads values for COA and UVC increased with an increase in temperature, confirming the observed reduction in inhibition efficiency with rise in temperature for the COA and UVC extracts. Activation energy (Ea) values for the inhibited solutions are generally higher than those for the uninhibited one, indicating a strong inhibitive action of the extracts by increasing the energy barrier for the corrosion process. The average values for COA, UVC and XYA are 82.35, 87.47 and 102.10 KJ/mol, respectively. The decrease of the inhibition efficiency with an increase in temperature observed indicates that the adsorption processes are physical in nature. Though the average Ea are greater than 80 KJ/mol stated by Ebenso [19], but the values of ΔGads obtained confirm that the adsorption of the inhibitor molecules onto the steel surface for all three extracts studied occurs via physical adsorption mechanism. Generally, values of ΔGads ≤ -20 KJ/mol signify chemical adsorption [20,21]. ΔGads values obtained were all negative, suggesting that the adsorption processes are spontaneous. Calculated values of Qads were mostly negative; indicating the adsorption of the studied extracts on steel surface is exothermic. The mean Qads value for the different plant extracts are -7.40, -2.14 and -32.18 KJ/mol for COA, UVC and XYA respectively.
Methanol extracts from COA, UVC and XYA were found to inhibit the corrosion of mild steel in HCl solutions. COA, UVC and XYA extracts showed inhibition efficiency of (83.7, 84.6 and 87.0) and (85.0, 62.5 and 76.1) for gravimetric (weight loss) and gasometric analysis respectively, previous studies have shown that this is possible [9,22,23]. The emphasis however, is not on whether the results from the two methods match but on the similar trend they both display within the range of imposed conditions of concentration, temperature and pressure. The corrosion inhibition efficiency increased with increase in the concentration of the extracts and decreased with the increase in time (days) and temperature. The mechanism of adsorption proposed for the plant extract is physical adsorption as it is evidenced from the activation parameters that the Ea of the blank is lower than the Ea in the presence of the plant extract, and the negative values of Qads and ΔGads indicate exothermic and spontaneous processes. Furthermore, the decreasing values of the Kads, from the Langmuir isotherms, as the temperature increased also assert that the mechanism of adsorption is by physical adsorption. The experimental data fit into the Langmuir adsorption isotherm.