Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2016) Volume 0, Issue 0
The thermodynamic modeling and optimization of the Gd-Sb and Gd-Bi-Sb systems were critically carried out by means of the CALPHAD (CALculation of PHAse Diagram) technique. The solution phases, liquid, bcc, rhomb and hcp(Gd) were described by the substitutional solution model. The compounds, β-Gd5(Bi, Sb)3, γ-Gd4(Bi, Sb)4, δ-Gd(Bi, Sb), δ′-Gd(Bi, Sb), ε-Gd16Sb39 and GdBi2 were treated as the formulae (Gd)m/m+n(Gd, Bi, Sb)n/m+n using two sublattice model in the Gd-Bi-Sb system. A self-consistent thermodynamic description of the Gd-Sb and Gd-Bi-Sb system were developed. The isothermal section at 300 K for the Gd-Bi-Sb system in the literature were reproduced in the present work.
Keywords: Gd-Bi-Sb system; Phase diagram; CALPHAD technique; Thermodynamic properties
In past years, the advanced magnetic materials and its potential as an energy savings technology greatly stimulated the interest of researchers and promoted the rapid development of magnetic refrigeration. Magnetic refrigeration could be realized by utilizing the heat release or absorption caused by the magnetic entropy change DSM of a magnetic material due to a magnetic field change DH [1]. The recent discovery of the giant magnetocaloric effect in Gd5(Si2Ge2) [1], gave further impulse towards the development of new materials [2]. Gd4Sb3 was one of the candidates for magnetic refrigerant near room temperature (266 K). Substitution of Bi for Sb in Gd4Sb3 increased its Curie temperature Tc up to 330 K [3]. The calculation of phase diagrams (CALPHAD) method, which was a powerful approach to save cost and short time during development of materials, effectively provided a clear guideline for material design. So in order to better understand the interactions of Gd and Sb with Bi and design high performance thermoelectric materials, it was important to study the thermochemical properties and the phase equilibria concerning the Gd-Bi-Sb system and to obtain the thermodynamic parameters of the system.
To obtain a thermodynamic description of a ternary system, the thermodynamic description of each involved binary system was necessary.
Gd-Bi system
The partial phase diagram of the composition range 0–60 at % Bi of the Gd-Bi binary system was firstly measured by Gambino [4], and three intermetallics, Gd5Bi3, Gd4Bi3 and GdBi, were reported in this system. In 1993, the phase diagram of the Gd-Bi system was re-determined and a new phase, GdBi2 was discovered [5]. The four intermetallics, Gd5Bi3, Gd4Bi3, GdBi2 and GdBi, were reported in the Gd-Bi binary system. All of the information of the Gd-Bi binary system was accompiled by Wang et al. [6]. The Gd-Bi binary system was assessed by Wang et al. [6]. Figure 1 presented the calculated phase diagram of Gd-Bi system using the thermodynamic description of Wang et al. [6]. The thermodynamic parameters obtained by Wang et al. [6] were adopted in the present work.
Figure 1: Calculated Gd-Bi phase diagram by Wang et al. [6].
Bi-Sb system
The Bi-Sb system was very simple and only two phases, liquid and rhomb(Bi, Sb) in this system, which was calculated by Dinsdale et al. [7]. The calculated the thermodynamic parameters [7] were accepted in the assessment of the Bi-Sb-Sn system [8] and the Ag-Bi-Sb system [9]. So the thermodynamic parameters obtained by Dinsdale et al. [7] were adopted in the present work. Figure 2 presented the calculated phase diagram of Bi-Sb system using the thermodynamic description of Dinsdale et al. [7]. Figure 2a presented the Bi-Sb system reproduced using the Thermo-Calc software [10] and Figure 2b presented the Bi-Sb system reproduced using the Pandat software [11].
Gd-Sb system
The Gd-Sb system was firstly determined in the concentrationl range 0-60 at% Sb by Gambino [4]. Later, Gerasimov [12] updated all the invariant equilibria temperatures. The whole composition and temperature ranges in the Gd-Sb phase diagram were established by Abdusalyamova et al. [13]. The four intermediate compounds, Gd5Sb3, Gd4Sb3, GdSb2, and GdSb were reported in this system. Recently, the previously called “GdSb2’’ phase was determined to be Gd16Sb39 [14], which was confirmed in the Gd-Bi-Sb ternary system [15]. So the Gd-Sb phase diagram was revised by Borzone et al. [14]. All of the information of the Gd-Sb system was compiled by Li et al. [16]. The updated phase diagram was assessed by Li et al. [16]. The thermodynamic parameters of the Gd-Sb system were well reproduced [16], but the parameters of Bcc(Gd) were difficult to reproduce the temperature of the Bcc(Gd). In the present work, to assess the Gd-Bi-Sb ternary system, the model of the five intermediate compounds, Gd5Sb3, Gd4Sb3, Gd16Sb39, α-GdSb (GdSb-low temperature as GdSb-LT) and β-GdSb(GdSbhigh temperature as GdSb-HT), was re-built to cosistent with other thermodynamic parameters of the relative compounds in the Gd-Bi system [6]. So the thermodynamic parameters of the five intermediate compounds were re-assessed in the present work. Figure 3 presented the calculated phase diagram of Gd-Sb system using the thermodynamic parameters of the five intermediate compounds calculated in this work and the thermodynamic parameters of the liquid assessed by Li et al. [16].
The information about the Gd-Bi-Sb system was very scarce. Recently, the phase diagram of the Gd-Bi-Sb ternary system had been determined at room temperature using X-ray powder diffraction and differential scanning calorimetry analysis by Jian et al. [15]. There were composed of six single-phase regions, six two-phase regions and one three-phase region. No ternary compound was reported in the Gd-Bi- Sb ternary system. The compounds, Gd5Sb3, Gd4Sb3 and GdSb (α-GdSb and β-GdSb) in the Gd-Sb system and their relative compounds, Gd5Bi3, Gd4Bi3 and GdBi in the Gd-Bi system were formed the continuous solid solutions, respectively. So the six single-phase regions are (Gd, Bi, Sb) solid solution (α phase), Gd5(Bi, Sb)3 solid solution (β phase), Gd4(Bi, Sb)3 solid solution (γ phase), Gd(Bi, Sb) solid solution (δ and δ′ phases), Gd16Ga39 (ε phase) phase and Gd (Hcp phase) phase in the Gd-Bi-Sb system. The maximum solid solubility of Gd in (Gd, Bi, Sb) at room temperature was determined to be 7.5 at %. The solid solubility of Gd in the other phases could not be detected. The above information was described in detail in the Ref. [15].
Unary phases
The Gibbs energy function for the element i (i=Gd, Bi, Sb) in the phase φ (φ = liquid, hcp, rhomb) was described as follows,
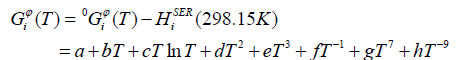 (1)
(1)
Where 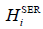 (298.15 K) was the molar enthalpy of the element i at 298.15 K in its standard element reference (SER) state, hcp for Gd, rhomb for Bi and Sb. The Gibbs energy of the element i(i=Gd, Bi, Sb),
(298.15 K) was the molar enthalpy of the element i at 298.15 K in its standard element reference (SER) state, hcp for Gd, rhomb for Bi and Sb. The Gibbs energy of the element i(i=Gd, Bi, Sb), 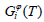 , in its SER state, was denoted by GHSERi, i.e.,
, in its SER state, was denoted by GHSERi, i.e.,
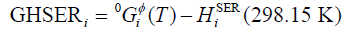 (2)
(2)
In the present work, the Gibbs energy functions were taken from the SGTE(Scientific Group of Thermodata Europe) pure elements database compiled by Dinsdale [17] and listed in Table 1.
Solution phases
In the Gd-Bi-Sb system, there are three solution phases, liquid, hcp, and rhomb. Their molar Gibbs energies are described by the following expression:
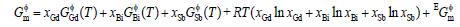 (3)
(3)
where R is the gas constant; xGd, xBi and xSb were the mole fractions of the pure elements Gd, Bi and Sb, respectively; 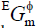 was the excess Gibbs energy, expressed by the Redlich-Kister polynomial [18].
was the excess Gibbs energy, expressed by the Redlich-Kister polynomial [18].
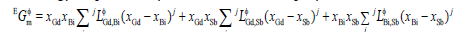
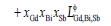 (4)
(4)
where 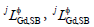 and
and 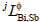 were the binary interaction parameters between elements Gd and Bi, Gd and Sb, and Bi and Sb, respectively. Its general form was
were the binary interaction parameters between elements Gd and Bi, Gd and Sb, and Bi and Sb, respectively. Its general form was
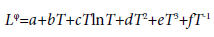 (5)
(5)
but in most case only the first one or two terms were used according to the temperature dependence of the experimental data. 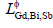 was the ternary interaction parameter expressed as:
was the ternary interaction parameter expressed as:
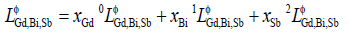 (6)
(6)
where 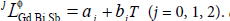 aj and bj were the parameters to be optimized in this work.
aj and bj were the parameters to be optimized in this work.
Intermetallic compounds
The compounds, Gd5Sb3, Gd4Sb3 and GdSb (GdSb-high temperature named as GdSb-HT and GdSb-low temperature named as GdSb-LT) in the Gd-Sb system and their relative compounds, Gd5Bi3, Gd4Bi3 and GdBi in the Gd-Bi system were formed the continuous solid solutions, respectively. So the three continuous solid solutions single-phase (named as Gdm(Bi, Sb)n), β-Gd5(Bi, Sb)3, γ-Gd4(Bi, Sb)3 δ-Gd(Bi, Sb) and δ′-Gd(Bi, Sb) in the Gd-Bi-Sb system were treated as two-sublattice model (Gd)m/m+n(Gd, Bi, Sb)n/m+n. In the Gd-Bi-Sb system, δ-Gd(Bi, Sb) and δ′-Gd(Bi, Sb) were the relative compounds, GdSb-high temperature (as GdSb-HT) and GdSb-low temperature (as GdSb-LT) in the Gd-Sb system. The Gibbs energy per mole of formula unit Gdm(Bi, Sb)n was given by the following expression:
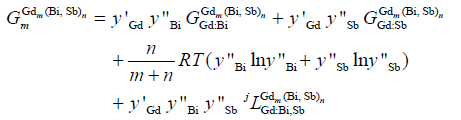 (7)
(7)
The parameter 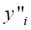 was the site fractions of Bi or Sb (i = Bi or Sb) on the second sublattices; the parameter
was the site fractions of Bi or Sb (i = Bi or Sb) on the second sublattices; the parameter 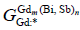 represented the Gibbs energies of the compound Gdm(Bi, Sb)n when the second sublattice was occupied by only one element Bi or Sb, respectively, which were relative to the enthalpies of pure rhomb for Bi and Sb in their SER state.
represented the Gibbs energies of the compound Gdm(Bi, Sb)n when the second sublattice was occupied by only one element Bi or Sb, respectively, which were relative to the enthalpies of pure rhomb for Bi and Sb in their SER state. 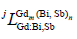 represented the jth interaction parameters (j=0) between the elements Bi or Sb on the second sublattice. The other binary intermetallic compounds, GdBi2 and Gd16Sb39 in the Gd- Bi and Gd-Sb system were treated as stoichiometric compounds, and were used as the two-sublattice model, which was consistented with the relative binary system [6,16].
represented the jth interaction parameters (j=0) between the elements Bi or Sb on the second sublattice. The other binary intermetallic compounds, GdBi2 and Gd16Sb39 in the Gd- Bi and Gd-Sb system were treated as stoichiometric compounds, and were used as the two-sublattice model, which was consistented with the relative binary system [6,16].
Assessment procedure
A general rule for selection of the adjustable parameters was that only those coefficients determined by the experimental values should be adjusted [19]. The assessment was carried out by means of the optimization module PARROT of the thermodynamic software Thermo-Calc [10], which could deal with various kinds of experimental information. A careful examination of thermodynamic descriptions of the Gd-Bi [6] and the Bi-Sb [7] systems were made. The thermodynamic optimization of the Gd-Sb and Gd-Bi-Sb system were carefully performed in this work. The thermodynamic parameters for the Gd-Sb system were optimized on the basis of the experimental information available in the experimental data. The thermodynamic parameters of liquid were taken from the assessed data [16]. The compounds, Gd5Sb3, Gd4Sb3, Gd16Sb39, α-GdSb and β-GdSb in the Gd-Sb system were assessed in the present work. The experimental results of Jian et al. [15] were given more weight during the process of optimization. The thermodynamic parameters for the Gd-Bi-Sb system were optimized on the basis of the experimental information available in the experimental data [15]. The experimental results of Jian et al. [15] were given more weight during the process of optimization. The thermodynamic parameters of liquid, bcc, rhomb and hcp in the Gd-Bi- Sb system, are obtained by a combination of the corresponding Gibbs energy functions from the assessments of the binary systems using Muggianu interpolation of binary excess terms [20]. The interaction parameters of the Gd-Bi and Bi-Sb system and the liquid of the Gd- Sb system were taken from the calculated data assessed by Wang et al. [6], Dinsdale et al. [7] and Li et al. [16]. The binary parameters of the compounds of the Gd-Sb system and the ternary parameters in the Gd- Bi-Sb system were optimized according to the experimental data [15].
The Gd-Bi and the Bi-Sb systems were assessed by Wang et al. [6], and Dinsdale et al. [7], respectively. Their thermodynamic descriptions were accepted in the present work and listed in Table 1. Figures 1 and 2 presented the Gd-Bi and Bi-Sb phase diagrams using the thermodynamic description of Wang et al. [6], and Dinsdale et al. [7], respectively. There was some different at low temperature using the Thermo-Calc Software [10] and the Pandat software [11] to reproduce the Bi-Sb phase diagram, as shown in Figures 2a and 2b.
Figure 3 presented the Gd-Sb phase diagram using the thermodynamic description of liquid assessed by Li et al. [16] and the calculated result of the compounds in the present work, and comparison with the experimental data [13]. Figure 4 presented the calculated standard enthalpies of formation in The Gd-Sb system at 300 K and comparison with the experimental data [14]. The reference states are hcp for Gd and rhomb for Sb. Reasonable agreement was obtained between the calculated results and the experimental data [14]. Figure 5 was the calculated enthalpies of mixing of liquid in the Gd-Sb system at 6000 K.
The calculated standard enthalpies of formation in the Gd-Sb system and comparison with the experimental data [14,21-25] were shown in Figure 4 and Table 2. The calculated invariant equilibria in the Gd–Sb system were listed in Table 3. As shown in the tables, a very good agreement was obtained between the calculated results and the experimental data [14]. The Gd-Bi-Sb system were optimized on the basis of the available experimental data [15]. The thermodynamic description of the Gd-Bi-Sb system obtained in the present work was shown in Table 1. Figure 6 were the calculated isothermal section at 300 K using the present thermodynamic description in comparison with experimental data [15] in the Gd-Bi-Sb system. Satisfactory agreements were obtained between the calculated results and the experimental data [15] in Figure 6. For the Bi-Sb phase diagram at low temperature (under 300 K), the rhomb was broken down into two component parts rhomb1 and rhomb2, as shown in Figure 2b. So there was some different at the rhomb phase side from the experiment data [15].
Figure 6: Calculated isothermal section of the Gd-Bi-Sb system at 300 K by the present thermodynamic description and comparison with the experimental data [15].
| Compound | Temperature, K | ΔfH | References |
|---|---|---|---|
| Gd5Sb3 | 300 | -110 | [16] |
| Gd5Sb3 | 673 | -141.9 | [23] |
| Gd5Sb3 | 300 | -103.664 | This work |
| Gd5Sb3 | 673 | -93.623 | This work |
| Gd4Sb3 | 300 | -116 | [16] |
| Gd4Sb3 | 673 | -145.1 | [23] |
| Gd4Sb3 | 300 | -118.254 | This work |
| Gd4Sb3 | 673 | -108.228 | This work |
| GdSb | 300 | -120 | [16] |
| GdSb | 300 | -131 | [25] |
| GdSb | 693 | -102.5 | [21] |
| GdSb-LT | 300 | -118.994 | This work |
| GdSb-LT | 753 | -106.672 | This work |
| GdSb-LT | 693 | -108.429 | This work |
| Gd0.52Sb0.48 | ˜753 | -137.1 | [22] |
| Gd16Sb39a | 300 | -78 | [16] |
| Gd16Sb39 | 300 | -87.697 | This work |
| Gd16Sb39 | 803 | -73.972 | This work |
| GdSb2 | 803 | -96 | [24] |
aThe nominal composition of GdSb2 was determined by Borzone et al. [14] to be Gd16Sb39 (70.91 at %Sb).
Table 2: Calculated formation enthalpy (kJ mole of atoms).
| Reaction | T (K) | x(Sb) | Reference | ||
|---|---|---|---|---|---|
| Liq. + bcc(Gd) →hcp(Gd) | 1505 | 0.065 | 0.025 | 0.02 | [13] |
| 1501 | 0.058 | 0.0191 | 0.0183 | [16] | |
| 1505 | 0.0821 | 0.0408 | 0.0387 | This work | |
| Liq. + hcp(Gd) →Gd5Sb3 | 1393 | 0.135 | 0.025 | 0.375 | [13] |
| 1390 | 0.1018 | 0.0402 | 0.375 | [16] | |
| 1393 | 0.1329 | 0.0685 | 0.375 | This work | |
| Liq. + Gd5Sb3 →Gd4Sb3 | 1913 | 0.35 | 0.375 | 0.4286 | [13] |
| 1917 | 0.3029 | 0.375 | 0.4286 | [16] | |
| 1913 | 0.3016 | 0.375 | 0.4286 | This work | |
| Liq. + Gd4Sb3 →βGdSb | 2043 | 0.389 | 0.4286 | 0.50 | [13] |
| 2048 | 0.3517 | 0.4286 | 0.50 | [16] | |
| 2043 | 0.3515 | 0.4286 | 0.50 | This work | |
| Liq. → GdSb-HT | 2403 | 0.50 | 0.50 | - | [13] |
| 2401 | 0.50 | 0.50 | - | [16] | |
| 2403 | 0.50 | 0.50 | - | This work | |
| GdSb-HT →GdSb-LT | 2113 | 0.50 | 0.50 | - | [13] |
| 2113 | 0.50 | 0.50 | - | [16] | |
| 2114 | 0.50 | 0.50 | - | This work | |
| Liq. + GdSb-LT →Gd16Sb39 | 1053 | 0.958 | 0.7091a | 0.50 | [13] |
| 1054 | 0.9665 | 0.709 | 0.50 | [16] | |
| 1053 | 0.9514 | 0.709 | 0.50 | This work | |
| Liq. + Gd16Sb39 →rhomb | 897 | >0.99 | 0.7091a | 1.0 | [13] |
| 903 | >0.99 | 0.709 | 1.0 | [16] | |
| 903 | 0.9984 | 0.709 | 1.0 | This work | |
aThe nominal composition of GdSb2 was determined by Borzone et al. [14] to be Gd16Sb39 (70.91 at %Sb).
Table 3: Invariant reactions of the Gd–Sb system.
The thermodynamic parameters in the Gd-Sb and Gd-Bi-Sb ternary system were critically evaluated from the experimental information available in the literature. A set of self-consistent thermodynamic parameters describing the Gibbs energy of each individual phase as a function of composition and temperature was derived.
This work was supported by National Natural Science Foundation of China (NSFC) (Grant No. 51264012), Jiangxi Province Science and Technology Support Program (Grant No. 20141BBE50006) and Jiangxi Province Natural Science Foundation (Grant No. 20142BAB206015), and the China Postdoctoral Science Foundation (Grant No. 2014M551712).