Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2017) Volume 7, Issue 1
A thermodynamic study of the enthalpies of adsorption of CO2 was conducted on polyethyleneimine 10k/ mesoporous silica (PEI-10k/MPS) and activated carbon (AC). These materials were chosen because of their high CO2 sorption capacities at about 85°C for PEI and about 20°C for the AC, therefore can capture CO2 with high efficiency in a wide temperature range. The absolute quantity of adsorbed CO2 as a function of equilibrium pressure at various temperatures was determined experimentally and fitted to isotherms of generalized Langmuir and Toth equations for PEI and AC, respectively. The adsorption of CO2 on PEI was favored with temperature revealing the endothermic nature of the process. On the other hand, CO2 adsorption on the AC was exothermic. The isosteric enthalpy of adsorption on PEI was about constant with CO2 loading at a value of 93 kJ.mol-1, confirming its chemical nature and corroboration with the Langmuir model. The corresponding isosteric enthalpy of the AC was in the range of ~-25 kJ.mol-1 and was continuously decreasing with CO2 loading; confirming the physisorption nature of the process and also that CO2/ CO2 interactions within the adsorption layer was significant.
<Keywords: Isosteric enthalpy of adsorption; Polyethyleneimine 10k/ mesoporous silica; Activated carbon
Increasing levels of CO2 emissions have precipitated a serious environmental concern. The main sources of CO2 emissions stem from natural gas streams and burned fossil fuels, so, attempts of removal of CO2 from these sources have been gaining widespread interest [1-3]. A wide range of technological techniques for capturing CO2 from natural gas streams has been proposed. These techniques include different physical and chemical procedures including absorption, adsorption, membranes and cryogenics. Chemical absorption by liquid amines is the most applicable industrial technology for CO2 scrubbing [4,5]. Several solid sorbents were also used. One subset is based on an inorganic – organic hybrid sorbents [6-8]. In most of these sorbents, the inorganic substrate was usually in the mesoporous form, where it provided both substantial pore volume and great surface area onto and into which the active organic groups were incorporated [9-12]. The most widely inorganic mesoporous support used till now is mesoporous silica [9-15]. Among the adsorption techniques, capturing CO2 on amine immobilized on solid sorbents has been considered as a great promising approach [16-19]. So far, amines immobilized on mesoporous silica (MPS) have shown to have the highest CO2 adsorptivity, high desorption rate, negligent corrosion problems, and low consumption of energy during regeneration [18,19]. Several amines supported mesoporous silica have been synthesized and studied [5,9-11,15,18,20]. Furthermore, when high molecular weight polyethyleneimine (PEI) is employed the volatilization and/or decomposition are expected to be minimal because of the relatively high melting temperature of such a high molecular weight material. Many other solid sorbents, e.g., activated carbons [21-24], zeolites [25,26], activated alumina [27-29], and membranes [30] have also been tested. Activated carbon showed great sorption capacity, however limited to use at lower temperatures and high pressures [31,32]. In general, mesoporous silica solid sorbent impregnated with PEI as well as AC are better solid sorbents compared to other solid materials as they are both lightweight and can effectively increase the volumetric density of captured gas.
In this study, we are interested in comparative studying the carbon dioxide adsorption on amine-functionalized mesoporous silica and AC in more detail. We have used a high pressure “Rubotherm magnetic suspension balance” to investigate the carbon dioxide adsorption on the two materials and constructed the adsorption isotherms from which the isosteric enthalpies of adsorption of carbon dioxide were evaluated.
Chemicals
Triblock co-polymer poly (ethylene oxide)-b-(propylene oxide)- b-poly(ethylene oxide) surfactant P123 (EO20PO70EO20, Mv=5800), polyethyleneimine, PEI 10K, Mn≈10 000), ethanol (v/v=90%), sodium silicate, acetic acid, ammonium fluoride were all purchased from Aldrich. In all experiments, deionized water was used.
Preparation of the polyethyleneimine PEI-10k/mesoporous silica (PEI-10k/MPS)
The mesoporous silica (MPS) support was prepared as reported previously [9,10,33]. Briefly 3.0 g of P123 was dissolved in acetic acid (3.0 g). Ammonium fluoride (0.3 g) and water (52 g) were added to the dissolved P123. The mixture temperature was fixed at 40°C. At the same temperature, a solution of sodium silicate (2.35 g) in water (40 g) was added into the surfactant solution under continuous stirring. The mixture was kept at 40°C for 24 h and then aged at 70°C for 24 h more. The product was filtered, followed and washed with DI water. The surfactant P123 was removed by heating in air at 560°C for 6 h.
Polyethyleneimine PEI-10k (10,000 g/mol) was impregnated into the mesoporous silica. Typically, 0.7 g of PEI-10k was added into 10 mL of ethanol. 1 g of mesoporous silica was added to the PEI-10k solution under stirring. The mixture was kept at room temperature for 12 h. The resultant slurry was dried at 100°C for 16 h. The obtained samples is referred to PEI 10k-MPS.
Granular activated carbon
Activated carbon was purchased from Sigma and used with no further treatment. Surface area was ~ 600 m2.g-1, pore volume 0.95 cm3.g-1 and particle size 12-40 (mesh).
CO2 sorption performance
Isothermal adsorption measurements were performed gravimetrically with DynTHERM SHP magnetic suspension balance (MSB) manufactured by Rubotherm GmbH (Bochum, Germany). The MSB provides the advantage of obtaining accurate results since it has a contactless weighing. The MSB apparatus has a resolution of 0.01 mg, measurement uncertainty less than 0.002%, and reproducibility within ±0.03 mg. All gases employed were obtained from a local vendor (Buzwair Scientific & Industrial Gases Qatar) with the following purities: Helium 99.9992%, and Carbon dioxide 99.99%.
The carbon dioxide adsorption test for each isotherm was conducted in four steps. First, a blank measurement was carried out with an empty sample container in helium ambience, and with stepwise increase in pressure from vacuum to 30 bar at constant temperature of 25°C. Second, after ~0.35 g of sample was loaded in the MSB, pretreatment was performed to degas the sorbent material at 110°C in vacuum for 60-90 minutes. Third, sorbent material isothermal density was determined by running a buoyancy measurement under inert condition with stepwise increase of pressure to 30 bar. Last, after the sample was again evacuated, adsorption measurements were carried out at 20°C, 25°C, 30°C and 35°C with high purity carbon dioxide, and with stepwise pressure increments to 20 bar for each isotherm. Adsorption equilibrium was assumed to occur when the pressure, temperature, and sample weight did not considerably vary; that took about one hour. The measured weight was periodically and automatically recorded in the operator computer, and the amount of gas adsorbed was determined using a spreadsheet. Acquisition of measurement data was made with RUBOTHERM System Control Software, where process parameters setting and monitoring were done.
CO2 adsorption performance
The total pore volume and the BET surface area of the MPS foam were 2.51 cm3.g-1 and 482 m2.g-1, respectively. The cumulative pore volume and surface area of the MPS foam calculated using the simplified Broekhoff-de Boer method were 2.5 cm3.g-1 and 750 m2.g-1, respectively. Previously, it was showed that the PEI material was immobilized inside the mesoporous foam cells with some on the external surfaces [20]. Nonetheless, the hierarchical porous structure between and within the sorbent particles was retained facilitating the diffusion of CO2 gas into the MPS foam [9,10]. Activated carbon is also a superior adsorbent because of its large number of micropores and high surface area. Therefore, it has been widely applied for gas adsorption.
Influence of pressure
It is well-established that the gas pressure is a crucial factor for the sorption capacity of various sorbents. Hence, PEI 10k/MPS and AC were selected to investigate the effect of the CO2 pressure on the sorption capacity using MSB with pure CO2 gas at pressures ranging from 0 to 25 bar. Figures 1A and 1B show CO2 adsorption isotherms obtained for the PEI 10k/MPS and AC, respectively. In these isotherms, the amount of CO2 adsorbed after equilibration, expressed as mg CO2/g of sorbent, was plotted against gas pressure in bar. For PEI-10k, the influence of pressure on CO2 adsorption has been studied by performing adsorption runs at 55, 65, 75 and 85°C. For AC, the adsorption isotherms were studied at 20, 25, 30 and 35°C.
It is clear from Figure 1A that most of CO2 (~70%) was adsorbed at pressure less than 1 bar, however, as shown in Figure 1B similar sorption capacity on the AC occurred at about 10 bars. The main difference between the two sorbents is due to the chemical nature of interaction of CO2 with the primary and secondary amines in the PEI molecules forming carbamates as represented by Eq. 1 and 2.
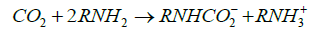 (1)
(1)
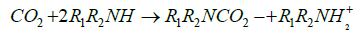 (2)
(2)
With AC, CO2 sticks to its surface via weaker forces, e.g. Van der Wall or dipole forces.
In both cases, the mass of adsorbed CO2, m, increased with the partial pressure, Pco2, in a nonlinear fashion as a consequence of the relatively high CO2 concentration in the gas stream. It is obvious that the equilibrium amount of CO2 absorbed, me, increased with the temperature for the PEI but decreased for the AC; implying the endothermic and exothermic nature of the adsorption process on PEI and AC, respectively.
Adsorption isotherm of PEI
The experimental values of the gas mass adsorbed on the PEI after reaching equilibrium, me was fitted best to the Langmuir equation:
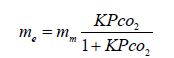 (3)
(3)
Where θ is the fractional surface coverage which equals me/mm, with me and mm the mass of gas adsorbed at pressure P after reaching equilibrium and the mass of gas covering a monolayer, respectively. The constant b, is indeed the adsorption equilibrium constant K (K=kads/ kdes), where kads and kdes are the specific rate constants for adsorption and desorption, respectively. Equation 3 is therefore rewritten as:
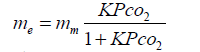 (4)
(4)
The constants of the Langmuir adsorption isotherm (mm and K) were obtained from fitting the measured me in Figure 1A to the Langmuir equation and the results are given in Table 1.
The correlation coefficients (R2) are in the 0.90-0.94 range, reflecting that the adsorption isotherm of CO2 on PEI could be well described by Langmuir adsorption model. The mm, the mass of gas covering monolayer of PEI sorbent, increased with temperature till reached a maximum value of ~273 mg CO2/ g sorbent at 85°C. The increase in the mm could be attributed to linearization of the PEI- 10 molecule by temperature, as it is originally branched non-linear molecule. By heating the PEI-10k, its molecules are expected to be more linear, therefore, having more of its amine groups exposed to CO2, consequently, increased its monolayer coverage as shown in Table 1. The constant K had a reverse behavior, i.e., decreased with increasing the temperature, implying that the gas desorption is kinetically faster at higher temperature. Although the kinetics of desorption is favored by increasing the temperature, but the sorption capacity was still increasing with temperature, as clearly shown in Figure 1A, indicating that the affinity to CO2 adsorption on PEI increases with temperature. It should be indicated that 85°C, was the temperature at which the maximum adsorption was recorded, after that temperature the sorption capacity was significantly declined, presumably, because the desorption kinetics prevailed.
| T, °C | R2 | K | mm |
|---|---|---|---|
| 55 | 0.901 | 8.367 | 180.2 |
| 65 | 0.905 | 7.594 | 197.91 |
| 75 | 0.923 | 6.861 | 218.5 |
| 85 | 0.940 | 5.66 | 237.63 |
Table 1: Langmuir adsorption isotherm data for the CO2 on PEI-10k/MPS.
Adsorption isotherm of AC
A more suitable isotherm for CO2 adsorption on AC, and generally for physisorption, is the Toth Isotherm [34-36]. The formula of the Toth isotherm describes gas/solid physisorption phenomena in a wide range of gas pressure. The Toth equation is written in the form
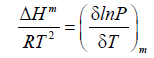 (5)
(5)
where P is the equilibrium pressure; m the mass of adsorbed gas; and qs, b, and t are isotherm parameters which are determined numerically. In this study, a nonlinear curve-fitting procedure was used to determine qs, b, and t. The parameters obtained from the best fit to the experimental data are summarized in Tables 2 and 3. The experimental data were well-fitted by the calculated isotherm for AC. From the R2 value it is, as previously reported [34], expected that the Toth adsorption isotherm describes well the physisorption phenomena of the CO2 on the AC.
| T, °C | R2 | qs | B | T |
|---|---|---|---|---|
| 20 | 1.0 | 584.05 | 1.32 | 0.46 |
| 25 | 1.0 | 576.32 | 1.39 | 0.46 |
| 30 | 1.0 | 583.59 | 1.53 | 0.47 |
| 35 | 1.0 | 600.15 | 1.60 | 0.45 |
Table 2: Toth adsorption isotherm data for the CO2 on AC
| m, CO2, mg/ g sorbent | R2 | ΔHm, kJ/mol |
|---|---|---|
| 173 | 0.978 | 93.4 |
| 184 | 0.983 | 93.4 |
| 190 | 0.985 | 93.9 |
| 196 | 0.986 | 94.4 |
Table 3A: Isosteric enthalpy of adsorption on PEI-10k.
| m, CO2, mg/ g sorbent | R2 | ΔH, kJ/mol |
|---|---|---|
| 66 | 0.998 | -29.8 |
| 137 | 0.984 | -26.5 |
| 254 | 0.990 | -22.5 |
| 275 | 0.997 | -19.2 |
Table 3B: Isosteric enthalpy of adsorption on AC.
Isosteric enthalpies of the adsorption
Isosteric enthalpies of adsorption (ΔHm) was calculated by the Clausius-Clapeyron equation for adsorption:
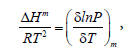 (6)
(6)
where P is the pressure, T is the temperature, and R is the gas constant. The isosteric enthalpy is a measure of the interaction between the adsorbate molecules and the adsorbent surface sites. The relationships between lnP and the inverse temperature for the PEI-10k and the AC are shown in Figures 2A and 2B, respectively. These are quite satisfactory linear relations with slope equals 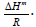
The isosteric enthalpy ΔHm as determined from the slops of Figures 2A and 2B are collected in Table 3.
The data in Table 3A clearly shows that isosteric enthalpy of CO2 adsorption on the PEI was about constant at a value of 93kJ/ mol, indicating that the CO2 adsorption on PEI is a typical Langmuir adsorption isotherm with a minor or no effect of the CO2-CO2 interaction on the extent of CO2 adsorption. The forces of interaction between the chemisorbed CO2 molecules, of course, is much weaker than the chemical adsorption bonds which were formed during the carbamate generation as signified from the values of ΔHm listed in Table 3A. The isosteric enthalpy of AC, on the other hand, was continuously decreasing with increasing the CO2 loading, revealing that the Toth adsorption isotherm is a suitable model for CO2 on AC. The change in the isosteric enthalpy with CO2 loading is explained due to the CO2-CO2 interaction forces which might compete with the weak physisorption forces associated with the CO2 adsorption on the AC surface. The change in the isosteric enthalpy with the gas loading could also be attributed due to a more heterogeneous surface structure.
In the comparison between the isosteric enthalpy of CO2 adsorption of PEI-10k/MPS and AC, it can be concluded that the significantly stronger amine adsorption sites existing on the PEI surface allow this material to capture CO2 at relatively high temperature and lower pressure. The strong CO2 chemisorbed bonds due to the carbamate formation made the CO2-CO2 interaction insignificant as revealed from the constancy of the isosteric enthalpy of adsorption at various CO2 coverage. On the other hand, the low enthalpy of adsorption (about -25 kJ mol-1) of CO2 on the AC as well as the corroboration with the Toth adsorption isotherm confirms the physisorption nature of the process. Furthermore, the decrease in the isosteric enthalpy with increasing CO2 coverage on the AC implied that CO2-CO2 interaction was significant and competed with the weak physisorbed CO2-AC bonds.
This paper was made possible by an NPRP Grant #5-1437-1-243 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors.