Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2020)Volume 8, Issue 1
Background: Oxidative stress is closely related to many diseases, especially autoimmune diseases. Many previous studies have shown that there is a close relationship between oxidative stress and the development of hyperthyroidism, but the oxidative stress indicators in patients with hyperthyroidism and the correlation between oxidative indicators and lipid metabolism remain controversial. The aim of this study was to investigate the levels of three oxidative stress indicators Diacron Reactive Oxygen Metabolites, Biological Antioxidant Potential and Superoxide Dismutase (DROM, BAP and SOD) in hyperthyroidism patients and healthy controls, and their relationship with the severity of hyperthyroidism and lipid metabolism.
Methods: 119 healthy individuals and 78 hyperthyroidism patients were included in this study. Automatic biochemical analyzer was used to detect three indicators of oxidative stress (BAP, SOD and DROM), three indicators of thyroid function (TSH, FT3 and FT4) and four indicators of lipid metabolism (TG, TC, LDL, HDL and fasting blood glucose) in patients with hyperthyroidism and healthy controls.
Results: The basic levels of BAP and SOD in peripheral blood were significantly lower in hyperthyroidism patients compared to healthy controls (Pï¼0.001), while the level of DROM was significantly higher in patients with hyperthyroidism compared with control subjects (Pï¼0.05). There was no significant correlation between the three oxidative stress indicators and metabolites of glucose and lipid. The level of DROM was negative correlated with TSH and positive correlated with FT3 and FT4.
Conclusion: Oxidative stress and antioxidant system play an important role in the pathogenesis of hyperthyroidism. In patients with hyperthyroidism, the level of oxidative stress products (DROM) was increased and the levels of antioxidant capacities (SOD and BAP) were decreased. BAP tend to be a better biomarker of antioxidant level in hyperthyroidism patients compared with SOD.
Oxidative stress, Reactive oxygen metabolites, Biological antioxidant potential tests, Hyperthyroidism, Lipid metabolism.
Oxidative stress reflects the imbalance between the system damage caused by reactive oxygen species and the damage caused by the detoxifying intermediates of the biological system [1]. When highly active molecules such as Reactive Oxygen Species (ROS) and reactive nitrogen species (RNS) are overproduced and exceed scavenging capacity of the body, it will lead to an imbalance between the oxidation system and the antioxidant system, thereby damaging all components of the cell, including proteins, lipids, and DNA. For example, oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA [2,3]. The relationship between oxidative stress and disease in humans has also been deeply investigated, such as atherosclerosis [4] Parkinson's disease [5], aging [6], cancer [7], respiratory muscle dysfunction [8], chronic obstructive pulmonary disease [9], diabetes [10,11], rheumatoid arthritis [12-14], osteoporosis [15,16], and so on. However, free radicals are extremely active and easily interact with other substances, making it difficult to measure their levels directly. Derivatives of Reactive Oxygen Metabolites (DROM) and biological antioxidant potential (BAP) are used to evaluate the overall level of oxidative stress by measuring the total active oxygen metabolites and total antioxidant capacity in peripheral blood, respectively [17,18]. Compared with detection of some free radical oxidative metabolites alone, DROM and BAP can evaluate the overall oxidative stress level more comprehensively [19,20].
Hyperthyroidism is a common clinical syndrome characterized by thyroid production and excessive secretion of free T3 or T4. Thyroid hormones, as the main hormones in the body that control metabolisms and respiratory rate, are associated with oxidative stress damage not only in their enhancement of metabolic, but also in their effects on antioxidant systems [21,22]. Excessive thyroxine (TH) is produced in patients with hyperthyroidism. TH accelerates energy metabolism by promoting intestinal glucose absorption and accelerating glucose oxidation and utilization [23,24], resulting in impaired glucose tolerance or aggravated diabetes. TH also accelerates the oxidative decomposition of lipids and proteins [3,25], which is the main cause of weight loss in patients with hyperthyroidism. There is little and controversial data on oxidant stress and antioxidant capacity in hyperthyroidism. Erdamar et al. showed that the serum levels of malondialdehyde (MDA), nitrite, vitamin E and myeloperoxidase (MPO) activity were increased in patients with hypothyroidism, and the activity of SOD was the highest in patients with hyperthyroidism. However, there was no significant differences between the hyperthyroid patients and controls [26]. By contrast, some other studies have shown that hyperthyroidism was characterized by increased levels of free radicals and peroxides, but decreased levels of antioxidant enzymes [27,28]. However, no study has examined the relationship among DROM, BAP values and hyperthyroidism concurrently.
The main purpose of this study is 1) to compare the differences of DROM, BAP and SOD levels between hyperthyroidism patients and normal controls, 2) to explore the correlation among the three indicators levels, disease severity and metabolites of lipid and glucose in hyperthyroidism patients, 3) to explore the role of three oxidative stress indicators in the evaluation of disease severity in hyperthyroidism patients. The originality of our study is the first time that we have investigated two comprehensive parameters (DROM, BAP) and the traditional antioxidant parameter (SOD) in patients with hyperthyroidism.
Patients
Hyperthyroidism group: 78 patients with hyperthyroidism from the outpatient department of Beijing Hospital from November 1, 2014 to March 31, 2015.
Inclusion criteria: the diagnosis criteria of hyperthyroidism were in accordance with “Guidelines for the diagnosis and treatment of thyroid diseases (2016 version)” [29]. Exclusion criteria: (1) history of asthma or other chronic lung disease; (2) history of treatment with antioxidants such as N-acetylcysteine, vitamin C and vitamin E; (3) symptomatic myocardium with new or recurrent disease, such as ischemia, severe arrhythmia, cardiac insufficiency; (4) history of malignant tumor, cirrhosis, chronic renal insufficiency, rheumatoid arthritis or other systemic inflammatory diseases; (5) neuromuscular disease or cognitive impairment; (6) participated in sports training in the previous 3 months. Healthy control group: no abnormalities in laboratory and imaging examinations. Exclusion criteria: The blood sample was visibly turbid or the patient had recently taken antioxidant drugs. All 119 participants were healthy volunteers, including 40 males and 79 females, all of whom were from the Physical Examination Center of Beijing hospital. The study was approved by the ethics committee of Beijing hospital, and all the subjects signed informed consent forms.
Determination of thyroid-related hormone and oxidative stress
5 ml of peripheral venous blood samples were collected from the participants and all the measurements were completed within three hours. FT3, FT4 and TSH were tested using SIEMENS ADVIA centaur and related reagents. The serum DROM level was detected by the method established by Cesarone et al. [30]. The serum SOD, BAP, total cholesterol (TG), triglyceride (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) and fasting blood glucose (G) were detected by an automatic biochemical analyzer (HITACHI Corporation, Japan).
Statistical Analysis
Statistical analysis was carried out with SPSS 20.0 software. The measurement data conforming to the normal distribution was described by “mean ± standard deviation”, and the non-normal distribution measurement data was described by median (interquartile range). The measurement data between the two groups was compared by t test (normal distribution data) or Mann-Whitney U test (non-normal distribution data); One-way ANOVA (normal distribution data) or Kruskal-Wallis H rank sum test (non-normal distribution data) were used to compare three or more groups. The Pearson correlation was used to analyze the correlation of normal distribution data, and the Spearman rank correlation was used to analyze the correlation of non-normal distribution data. P<0.05 was considered to be statistically significant.
A total of 78 patients with previous or initial diagnosis of hyperthyroidism were enrolled in the study, including 18 males and 60 females with an average age of 45.69 years. There were 119 cases in the healthy control group, including 40 males and 79 females, with an average age of 41.39 years old. The level of oxidative indicator DROM was much higher in hyperthyroidism individuals than that in healthy controls, while the levels of antioxidant indicators SOD and BAP were significantly lower than those in the control group. Hyperthyroidism patients have an elevated level of fasting blood glucose and reduced level of lipid metabolites than those in healthy controls. Details were shown in Table 1.
| Factor | Hyperthyriodism | Healthy Control | P value |
|---|---|---|---|
| Total | 78 | 119 | >0.05 |
| Male | 18 | 40 | - |
| Female | 60 | 79 | - |
| Average age (y) | 45.69 ± 13.08 | 41.39 ± 13.07 | <0.05 |
| SOD (unit) | 144.26 ± 20.32 | 156.45 ± 12.24 | <0.001 |
| DROM (mmol/L) | 97.92 ± 23.14 | 86.07 ± 18.49 | <0.05 |
| BAP (mmol/L) | 2.02 ± 0.11 | 2.75 ± 0.13 | <0.001 |
| G (mmol/L) | 5.43 ± 0.57 | 4.93 ± 0.45 | <0.001 |
| TC (mmol/L) | 4.29 ± 0.78 | 4.48 ± 0.47 | <0.05 |
| TG (mmol/L) | 1.17 ± 0.73 | 1.22 ± 0.33 | <0.05 |
| LDL (mmol/L) | 2.44 ± 0.69 | 2.61 ± 0.47 | <0.05 |
| HDL (mmol/L) | 1.39 ± 0.33 | 1.59 ± 0.3 | <0.001 |
Table 1: Laboratory parameters of oxidative stress and glucose, lipid metabolism in hyperthyroidism and healthy controls.
Considering that the patients were studied in both the hyperthyroid and euthyroid states during study, we divided the patients into hyperthyroid group and euthyroid group to explore the effects of antithyroid therapy on oxidative stress, glucose and lipid metabolism in hyperthyroidism patients. Of the 78 patients who participated in the study, 33 were newly diagnosed with hyperthyroidism state and 45 were in euthyroid state due to the antithyroid treatment. Compared with the hyperthyroidism group, DROM was significantly decreased and lipid metabolites TC, TG, LDL and HDL were significantly increased in patients with eythyroid state, but there was no significant difference in these five indicators between patients with eythyroid state and healthy controls. Another interesting finding was that compared with healthy controls, patients in euthyroid state still had lower levels of BAP and SOD and slightly higher fasting blood glucose level after antithyroid therapy. Details are shown in Table 2.
| Factor | Hyperthyriodism | Euthyroid | Healthy Control |
|---|---|---|---|
| Hyperthyroid | |||
| Total | 33 | 45 | 119 |
| Male | 9 | 9 | 40 |
| Female | 24 | 36 | 79 |
| Average age (y) | 46.73 ± 14.29 | 44.93 ± 12.22 | 41.39 ± 13.07 |
| SOD (unit) | 138.85 ± 21.61*** | 148.22 ± 18.57# | 156.45 ± 12.24※ |
| DROM (mmol/L) | 112.61 ± 27.13*** | 87.16 ± 11.02### | 86.07 ± 18.49 |
| BAP (mmol/L) | 2.01 ± 0.11*** | 2.02 ± 0.11 | 2.75 ± 0.13※※※ |
| G (mmol/L) | 5.51 ± 0.50*** | 5.37 ± 0.61 | 4.93 ± 0.45※※※ |
| TC (mmol/L) | 3.78 ± 0.75*** | 4.67 ± 0.56### | 4.48 ± 0.47 |
| TG (mmol/L) | 0.94 ± 0.46** | 1.34 ± 0.84# | 1.23 ± 0.33 |
| LDL (mmol/L) | 2.31 ± 0.61*** | 2.66 ± 0.68## | 2.61 ± 0.47 |
| HDL (mmol/L) | 1.25 ± 0.30** | 1.49 ± 0.32## | 1.59 ± 0.3 |
| TSH (mIU/ml) | 0.02 ± 0.058 | 2.86 ± 0.59### | - |
| FT3 (pmol/L) | 7.33 ± 2.83 | 3.04 ± 0.44### | - |
| FT4 (pmol/L) | 2.47 ± 0.78 | 1.21 ± 0.28### | - |
| Data represent mean ± SEM. | |||
| *p<0.05, hyperthyroid group vs. healthy control group | |||
| **p<0.01, hyperthyroid group vs. healthy control group | |||
| #p<0.05, hyperthyroid group vs. euthyroid group | |||
| ##p<0.01, hyperthyroid group vs. euthyroid group | |||
| ※p<0.05, euthyroid group vs. healthy control group | |||
| ※※p<0.01, euthyroid group vs. healthy control group | |||
Table 2: Laboratory parameters of oxidative stress, glucose and lipid metabolism and thyroid function in the hyperthyroid group, euthyroid group and control group.
We used Pearson correlation analysis to further explore the correlation between oxidative stress, thyroid function, and glycose and lipid metabolism. According to the results shown in Table 3, it can be assumed that there was no significant correlation between SOD, BAP and TSH, FT3 and FT4, but DROM was negatively correlated with TSH and positively correlated with FT3 and FT4. SOD and BAP were positively correlated with fasting blood glucose, but the correlation was extremely weak. Except for the weak correlation between BAP and HDL, there was no significant correlation between the expression levels of SOD and DROM and the expression levels of lipid metabolites. See Table 3 and Figure 1 for specific information.
| Age | TSH | FT3 | FT4 | G | TC | TG | LDL | HDL | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SOD | ρ | -0.612 | 0.048 | -0.093 | -0.093 | -0.366 | 0.015 | 0.023 | 0.027 | 0.059 |
| P | <0.001 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | |
| BAP | ρ | -0.223 | -0.034 | -0.025 | -0.07 | -0.426 | 0.071 | 0.133 | 0.102 | 0.231 |
| P | <0.005 | >0.05 | >0.05 | >0.05 | <0.001 | >0.05 | >0.05 | >0.05 | < 0.001 | |
| DROM | ρ | 0.196 | -0.422 | 0.489 | 0.461 | 0.136 | -0.137 | -0.124 | -0.100 | -0.077 |
| P | <0.05 | <0.001 | <0.001 | <0.001 | >0.05 | <0.05 | >0.05 | >0.05 | >0.05 |
Table 3: Pearson correlation analysis of oxidative stress indicators and thyroid function, glucose and lipid metabolites
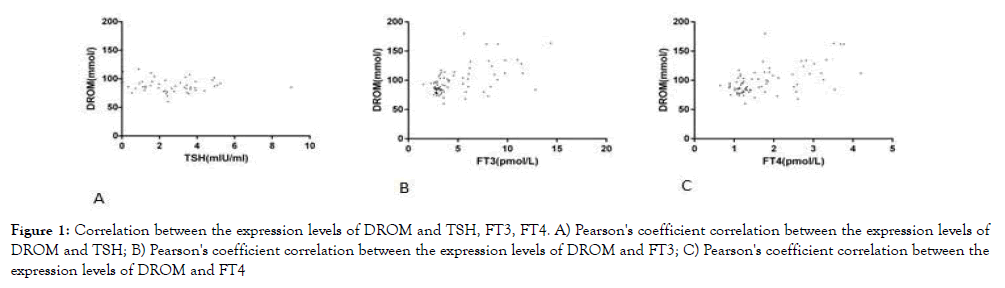
Figure 1. Correlation between the expression levels of DROM and TSH, FT3, FT4. A) Pearson's coefficient correlation between the expression levels of DROM and TSH; B) Pearson's coefficient correlation between the expression levels of DROM and FT3; C) Pearson's coefficient correlation between the expression levels of DROM and FT4
Hyperthyroidism is caused by excessive secretion of thyroid hormones, and the main symptoms are metabolic syndrome, goiter and eye signs. The diagnosis is mainly based on the determination of thyroid hormone, which was characterized by the decrease of Thyroid Stimulating Hormone (TSH) and the increase of thyroid hormone levels (FT3 and FT4) [29].
ROS in the body are mainly derived from the adenosine triphosphate (ATP) synthesized in the mitochondria, electron transport chain and oxidase reaction on the microsomal membrane. All cells produce ROS, while those with strong aerobic metabolism such as neutrophils, smooth muscle cells and vascular endothelial cells produce more ROS [30,31]. Previous studies have shown that hyperthyroidism can increase free radicals and lipid peroxides produced by oxidative stress [27,32,33]. Thyroid hormones regulates the energy metabolism of mitochondria by increasing the activity and concentration of sodium-potassium ATPase as well as increasing the permeability of sodium and potassium ions [34-36].
The DROMs test detected organic hydroperoxides mainly generated from the oxidation of lipids, but also proteins and nucleic acids [17]. It can provide a simple, inexpensive and practical way to identify subjects with a high level of oxidative stress [30]. When the mediumhigh oxidative stress is reached, the antioxidant defense systems fail to maintain DROM levels and it will be increased significantly [37]. The BAP level reflects total antioxidant capacity, including proteins, uric acid, bilirubin, ascorbic acid and α-tocopherol, and it is characterized by a high analytic performance [18,20]. However, there are relatively few studies on the role of DROM, BAP and SOD in oxidative stress in patients with hyperthyroidism.
In the present study, the levels of BAP and SOD in peripheral blood of patients with hyperthyroidism were significantly lower than those in healthy controls, while the level of DROM was higher than that in healthy controls. DROM can be decreased to a state similar to that in healthy control after treatment, while SOD and BAP can be partially increased but still lower than that in healthy control group. So far, a lot of work has focused on the oxidative stress in patients with hyperthyroidism and found that oxidative metabolites were increased in these patients [38- 40]. When the production of free radical increased, the activities of antioxidant enzymes such as SOD increased compensatively in order to scavenge free radicals. However, our present study has shown that the antioxidant activities of BAP and SOD were lower than those in the healthy control group, which was consistent with some other studies [41-44]. This may be related to the SOD consumption or the reduction in synthesis of antioxidants. Bianchi et al. confirmed that the presence of oxidative stress and decreased anti-oxidant metabolites in hyperthyroid patients, which were corrected in euthyroidism, without any influence of thyrostatic drugs per se. They also suggested that nutritional support with antioxidant agents, which are defective during hyperthyroidism [28]. Besides, different tissues have different sensitivity to thyroid hormones. For example, the level of lipid peroxidation was found to be increased in hindlimb muscles [45], but unchanged in heart [46] and even decreased in liver [47] from hyperthyroid mice. This may also be one of the reasons for the contradiction between the different research results.
Previous studies have studied the ability of biological antioxidants under oxidative stress in a variety of diseases, such as acute phase of Kawasaki disease, metabolic syndrome, hemodialysis patients [48-50]. All these results confirmed the good response of BAP to biological antioxidant in diseases. In this study, as shown in Figure 2. 2B, the BAP levels in most patients with hyperthyroidism were below the normal reference range, regardless of their age and gender. Importantly, BAP measurement can be performed with venous serum, and it examines the blood concentration of antioxidants as agents that can reduce iron from the ferric (Fe3+) to ferrous (Fe2+) form. It provides more reliable results than established antioxidant markers because the interactions between individual antioxidants are taken into account and the effects of unknown antioxidants are also taken into consideration. It is also easy to perform, inexpensive, utilizes small equipment, and quick [48-50]. Taken together, BAP is better than SOD to reflect the antioxidant levels in hyperthyroidism patients.
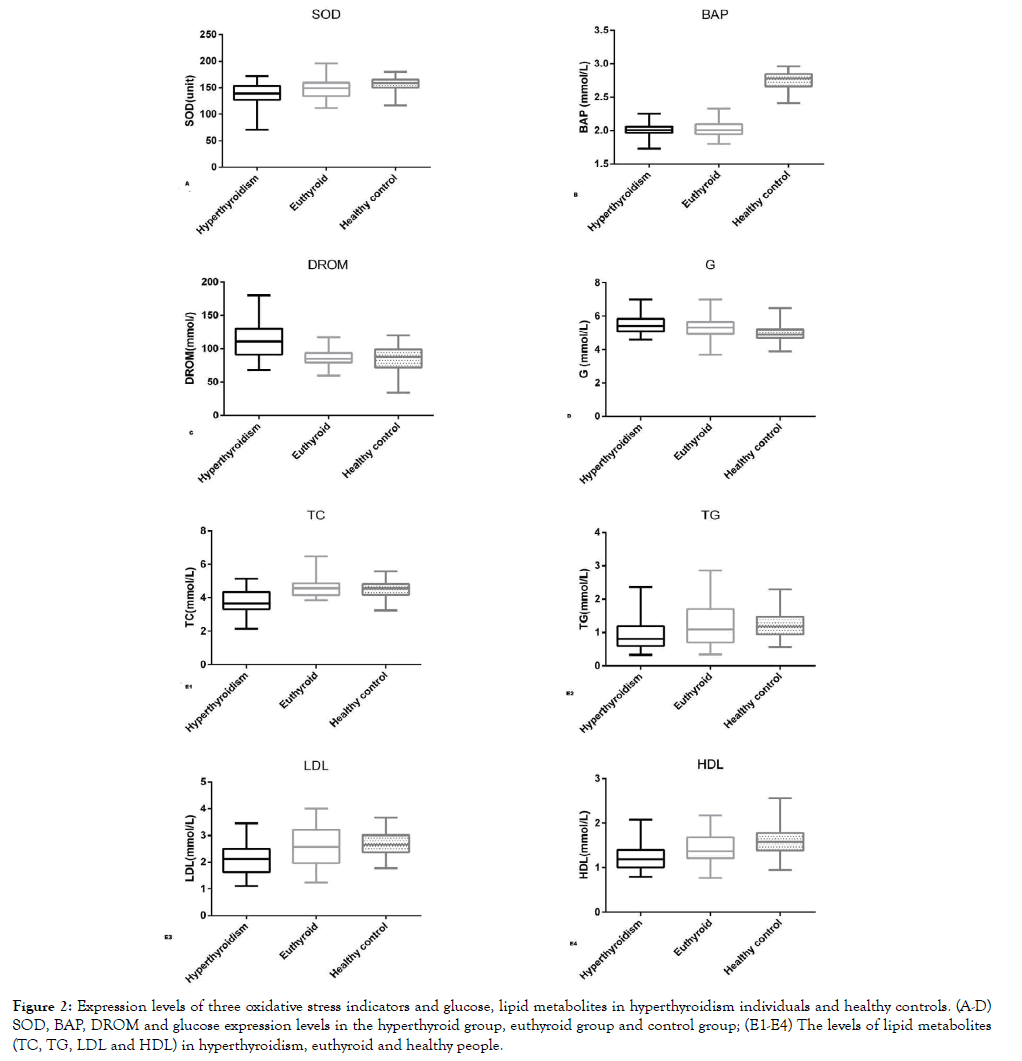
Figure 2. Expression levels of three oxidative stress indicators and glucose, lipid metabolites in hyperthyroidism individuals and healthy controls. (A-D) SOD, BAP, DROM and glucose expression levels in the hyperthyroid group, euthyroid group and control group; (E1-E4) The levels of lipid metabolites (TC, TG, LDL and HDL) in hyperthyroidism, euthyroid and healthy people.
The levels of fasting blood glucose and blood lipid metabolites have also changed with the development of hyperthyroidism. Many studies have shown that increased non-oxidized glucose in patients with hyperthyroidism leads to increased production of lactic acid and increased hepatic glucose output [23,24]. On the other hand, thyroxine stimulates the expression of glucose transporter 2 (GLT2) on the surface of liver cells [51-54]. In addition, the increase of lipid oxidative decomposition leads to increase of free fatty acids [53]. All of these can cause an increase in liver glycose output, leading to an increased blood glucose or a deterioration of preexisting diabetes mellitus in hyperthyroidism patients. Our results showed that fasting blood glucose levels in hyperthyroidism patients are significantly higher than in healthy controls, and some patients had hyperglycemia or diabetes. Although received treatment, the fasting blood glucose of euthyroid patients are still higher than that of the healthy control group. BAP has the strongest correlation among the three oxidative stress indicators with blood glucose.
In addition to changes in blood glucose, lipid metabolisms also undergone significant changes in hyperthyroidism patients. Lipid synthesis and blood lipid levels decreased in patients with hyperthyroidism because of increased lipid oxidation rate and decreased lipid synthase activity [54,55]. Our results are in good consistent with previous studies [25,56-60]. For example, hyperthyroidism patients have a decreased level of blood lipid and can rise to normal level when they reach euthyroid state. However, the correlation between blood lipids and the three oxidative stress indicators are very weak. Although lipid metabolites are significantly reduced, it seems that blood lipid level does not reflect well the increased oxidative stress in hyperthyroidism patients.
We also explored the relationship between these three oxidative stress indicators and thyroid function, but there was no significant correlation between BAP, SOD and thyroid function except that DROM was negatively correlated with TSH and positively correlated with FT3 and FT4.
There are several limitations in our research. First of all, we did not take into account lifestyle factors, such as smoking [58], nutrient intake [59], and alcohol intake [60], which are known to be associated with oxidative stress. Second, the patients included had no detailed clinical information, nor did they have information on the treatment method of patients with hyperthyroidism and course of illness. In the next study, we will further expand the sample, conduct a more detailed analysis of the patient's habits (smoking, drinking) and thyrostatic drugs, and collect more indicators such as inflammatory factors, and other indicators of the body's oxidative stress levels such as GPx (glutathione peroxydase), catalase (CAT), MDA etc.
Based on our findings, antioxidant agents are defective in hyperthyroidism patients, and we would suggest that they should take some antioxidant drugs during treatment to reduce the damage caused by excessive production of reactive oxygen species to cellular components.
This study was supported by the National Key Research and Development Program of China under Grant 2017YFC1309800; CAMS Innovation Fund for Medical Sciences under Grant 2018- I2M-1-002, National Natural Science Foundation of China under Grant 81870048 & 81602321.
The authors report no conflict of interest.
Citation: Yang P, Ying L, Li H, Wang X, Jia X, Zou L, et al. (2020) Three Indicators of Oxidative Stress in the Evaluation of Hyperthyroidism. Adv Tech Biol Med. 8:268. doi: 10.35248/2379-1764.20.8.268
Received: 14-Mar-2020 Accepted: 02-Apr-2020 Published: 09-Apr-2020 , DOI: 10.35248/2379-1764.20.8.268
Copyright: © 2020 Yang P et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This study was supported by the National Key Research and Development Program of China under Grant 2017YFC1309800; CAMS Innovation Fund for Medical Sciences under Grant 2018-I2M-1-002, National Natural Science Foundation of China under Grant 81870048 & 81602321.