Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2021)
The magnitudes of Anti-Retroviral Treatment (ART) failure for adult people living with HIV (PLWH) were exhaustively studied; however, time to treatment failure among seropositive children was overlooked, and this study aimed to assess time to first-Line Antiretroviral treatment Failure for seropositive children.
Methods: Facility-based retrospective follow-up study was conducted since 1 January 2016-30 December 2020. EPI- DATA version 3.2 and STATA/14 software were used for data entry and analysis, respectively. Proportional hazard assumption was checked for each variable and no variable was found with Schoenfeld residual test <0.05. Categorical variables at bi-variables Cox regression were assessed for candidates transferred at P-value <0.25 for multivariable Cox regression to claiming predictors associated for TB incidence rate at 95% CI at P<0.005.
Results: A total of 710 recorded of ART files were reviewed with 96 children (13.5%) (95% CI: 11.2, 16.3) had developed treatment failures. The overall incidence rate of treatment failure was found 4.098 (95% CI: 3.35 to 5.02) per 1000 Person Month. Children being orphaned (AHR: 4.3, 95% CI: 2.17, 7.7), WHO stage III and IV (AHR: 3.5, 95% CI: 1.8, 7.4), Poor ART adherence 3.27 (AHR:3.27, 95% CI:1.54, 4.8), ART follow-up duration ≥ 72 months (AHR: 2.28, 95% CI: 1.2, 5.2), Missed CPT 6.7 (AHR-6.7; 95% CI: 3.6, 8.4), AZT-3TC-NVP 6.5 (AHR=6.5; 95% CI: 3.2, 18.2), AZT-3TC-EFV 2.9 (AHR=2.89, 95% CI: 2.89, 10.1) were associated with treatment failures.
Conclusion: Sixty-two percent of treatment failures were occurred after 72 months of ART follow up with a higher incidence of treatment failures, which is unacceptable as compared with slandered reference <10%. Being a seropositive child ≥ 70 month on ART, WHO stage III and IV, ART regiment (AZT-3TC-NVP and AZT-3TC-EFV), Poor ART adherence, missing CPT, and orphanages were associated with treatment failure.
HIV/AIDS; Antiretroviral therapy; Treatment failure; Children
Acquired Immune Deficiency Syndrome (AIDS) is a viral infection caused by the Human Immunodeficiency Virus (HIV) that weakens the immune system and makes the body susceptible to secondary and opportunistic infections [1]. The disease continues to be a major global health priority, where more than 2 million children worldwide are infected with HIV, approximately 90% of whom live in sub-Saharan Africa. Sub-Saharan Africa has the highest burden of HIV/AIDS worldwide [2-4]. It remains the most heavily affected region, accounting for 71% of all new HIV infections and an estimated 430,000 new HIV infections occurred among children under the age of 15. Antiretroviral Therapy (ART) coverage rose from 7% in 2003 to 42% in 2008, with especially high coverage achieved in eastern and southern Africa (48%) [4-6].
AIDS develops very rapidly among Infants and young children living with HIV, have a high risk of poor outcomes, with up to 52% of children born with HIV dying before the age of 2 years and one-third before year one in the absence of any intervention [7]. 120,000 children died of AIDS-related causes in 2016 [4]. A serious challenge for children with HIV is maintaining long-term adherence to treatment regimens, and thus virological suppression and prevention of treatment failure [7]. The long duration of therapy needed for HIV-infected children requires maximal efficacy, minimal toxicity, and prevention of development of drug resistance which requires consideration of ways to minimize the occurrence of resistance and treatment failure [8]. Children, more so than adults, are particularly vulnerable to resistance, especially in resource-limited countries, due to a frequently high Viral Load (VL) before the start of treatment, unavailability of adapted drug formulations, frequent poor adherence, and limited VL access [5,9]. Recommending potent and effective second-line regimens for infants and children is difficult because treatment options are largely non-existent in most low-income countries [10-12]. Consequently, this challenge emphasizes the significance of choosing potent first-line regimens and the necessity to make maximal efforts to ensure increased durability of first-line regimens and early consolidation for virtual elimination of mother-to-child transmission of HIV is an indispensable way for prevention of treatment failures [5,13]. Pieces of evidence showed that different predictors affect treatment failure. The most common predictors are age, gender, being orphan, time from ART initiation, poor adherence to ART failure, nevirapine (NVP)-based regimen, drug side-effects, drug toxicity, nutritional status, pretreatment CD4 count,clinical stage and tuberculosis co-infection [11,12,14-30].To reduce the problem of HIV/AIDS, ART was endorsed in 2003, and in January 2005, free ART was launched in Ethiopia [10,14]. In Ethiopia, despite the integrated implementation of antiretroviral therapy since 2005, HIV remains public health concern. Managing and detecting ART treatment response is important to monitors effectiveness of therapy [31]. Even though the magnitude of ART treatment failure for seropositive children was well documented in Ethiopia, the specific time when treatment failed during follow-up and its predictors are lacking.
Study design and setting
An institution-based retrospective follow-up study was conducted to assess treatment failure of seropositive children in three general hospitals from January 1/2016 to December 31/2020. The one i.e Pawe hospital is located 565 km far from Addis Ababa in the Metekel zone at Benishangul Gumuz regions [27]. The two hospitals (Chagni and Jawi Hospitals) were located in the Amhara regions, North West of Ethiopia. All these health facilities provide health care services for an estimated 2621 total catchment populations [3,32]. All HIV-infected children who were under 15 years of age, who initiated ART between January 30, 2016, and January 2020, and who took first-line ART for at least five months were included in this study were considered as source population. Those charts of children who have not at least one of the three key failure indicator variables (WHO clinical stage, CD4 count, and VL) were excluding (Figure 1).
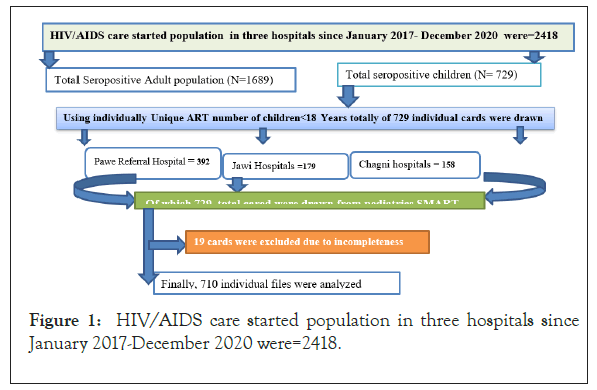
Figure 1: HIV/AIDS care started population in three hospitals since January 2017-December 2020 were=2418.
Sample size determination and sampling techniques
The sample size was calculated by using the formula for survival analysis considering two-sided significance level (α=5%), Za/2=Z value at 95% confidence interval=1.96, power (ZB)=80%, and P=% cumulative occurrence of death rate, 1.65 HR [33].
The final sample size (n)=Event/P(event)=(Za/2+Zb)2/θ2p(1- p)=(Za/2+Zb)2/p(1-p)(lnHR)2 [33].
Where, Alpha (α)=0.05, Beta (β)=0.2, AHR: Hazard ratio; E: Number of event; N(sample size)=E/P(E), where P(E)=Probability of Event, and P=cumulative occurrence of treatment failure used valued from reference for sample size calculation [8]. The final sample size was determined as 509.45 after adding 15% contingency for incompleteness. However, from January 30, 2016, up to 30 December 2020 there were 729 multi charts reviewed and all 729 included for final analysis since it is manageable.
Operational words
Event: Any types of treatment failure after initiation of first-line ART (clinical, Immunological and virological failure).
Treatment failure is categorized as a clinical, immunological, and virological failure and defined as follows [14,23].
Clinical failure: New or recurrent clinical event indicating advanced or severe immune deficiency (WHO clinical stage 3 and 4 clinical conditions with exception of TB) after 6 months of effective treatment.
Immunological failure: Persistent (at least 2 CD4 measurements) CD4 levels below 200 cells/mm for children younger than 5 years and, CD4 levels below 100 cells/mm for older than 5 years.
Virological failure: Viral load above 1000 copies/mL based on two consecutive viral load measurements in 3 months, with adherence support following the first viral load test [7,14,34].
Data collection instruments
A structured English version checklist was developed and used for data extraction from the patients' medical records on the Federal Ministry of Health Antiretroviral Therapy (ART) follow-up form and pediatrics HIV intake form. These records included; clients' age, gender, weight, height, duration of follow-up, clinical and laboratory data (WHO stage, opportunistic infections, CD4 count and white blood cell count), the ART regimen and prophylaxis, and adherence on a structured data retrieval form. Trained health professionals (nurses) working in the ART clinic were recruited as data collectors and supervisors.
Data collection procedures and quality assurance
To assure the quality of data, data collectors and supervisors were trained about how and what information they should collect from the medical records for one day. The checklist was pretested on 5% (21) of randomly selected charts, which were not included in the actual study. After the pretest, necessary modification of the data collection tool was made. Strict follow-up and supervision were carried out during data collection by principal investigators and feedback was given daily. The completeness of each collected data was audited at the end of each day by the principal investigator and supervisor. Whenever there appear incompleteness and uncertainty of recording, the filled information formats were cross- checked with source data soon. Individual records with incomplete data during data collection were excluded.
Data processing and analysis
The collected data were entered using Ep-data version 4.2 statistical software and exported to STATA (SE)R-14 version statistical software for further analysis. The proportional hazard assumption was checked for each variable and no variable was found with Schoenfeld residual test <0.05. The Kaplan-Meier survival curves and log-rank test were performed to compare significant experience among survival/death study participants. Variables with P-value <0.25 in bi-variable Cox regression analysis were included into multivariable Cox regression model. Finally, a variable with an Adjusted Hazard Ratio (AHR) it 95% Confidence Interval (CI) at P-value <0.05 were considered as significant predictor for inpatient mortality admitted children. The model fitness was checked using the Nelson-Aalen and Cox-Snell residuals test.
Ethical approval and consent to participant
Ethical clearance and ethical approval were obtained from the research Intuitional Review Board (IRB) of Debre Markose University with Reference (Refill No: DMU IRB-984/118/13). As the study was retrospective, the IRB waived that the research could be done based on record review without contacting patients. A cooperation letter was obtained from Debre Markose University, College of Health Science.
Socio-demographic characteristics
From the beginning of 2016 to the end of 2020, a total of 729 children below 18 years of age were started ART. Three hundred ninety-two (392) cases were from Pawe Referral hospital, 158 were from Changi General Hospital and the rest 179 were from Jawi General Hospitals. During the data extraction process, 11 HIV- positive children's medical record charts were excluded due to incomplete documentation and lack of important variables (were excluded from analysis because of missing data on at least one of the 3 failure indicator variables. Out of the included participants, slightly more than half, 379 (53.38%) were female and the mean age was 4.88(SD=± 2.97) years. The majority, 369 (51.84%) of study participants were from the urban area and the majority of 306 (43.10%) caregivers' religion was orthodox followers. Less than three to nine, 231 (32.89%) and 153 (21.74%) of the caregivers were daily laborers and merchants, respectively. Nearly half of 348 (49.15%) of the care both parents were alive with their biological children (Table 1).
| Socio-demographic characteristics | Categories | Number | Frequency |
|---|---|---|---|
| Age characteristics | <1 year | 42 | 5.92 |
| 1 to 4 | 257 | 36.2 | |
| 5-16 | 411 | 57.89 | |
| Sex | Male | 331 | 46.63 |
| Female | 379 | 53.38 | |
| Resident | Urban | 369 | 51.97 |
| Rural | 341 | 48.03 | |
| Parental status | Both lived | 342 | 48.24 |
| Paternal orphan | 161 | 22.71 | |
| Maternal orphan | 129 | 18.19 | |
| Both orphan | 77 | 10.86 | |
| Occupational status | Farmer | 122 | 17.18 |
| Merchant | 153 | 21.55 | |
| Government employer | 173 | 24.37 | |
| Daily laborer | 233 | 32.82 | |
| Others | 29 | 4.08 | |
| The religion of caregiver | Orthodox | 294 | 43.1 |
| Muslim | 200 | 22.39 | |
| Protestant | 162 | 24.51 | |
| Catholic | 54 | 7.13 | |
| Family size | <=2 | 223 | 31.41 |
| 3-6 | 456 | 64.23 | |
| >=6 | 31 | 31.37 |
Table 1: Socio-demographic characteristics of children on ART at Northwest Ethiopia referral hospitals, since (2016-2020) (N=710).
Baseline medical opportunistic infection
Of the total 710, nearly two in five 258 (36.34%) of participants had at least one form of medical opportunistic infection developed. The most frequent comorbidities listed were as follows: Severe acute malnutrition 81 (31.5%), TB 49 (28%), PCP 29 (11%), diarrhea 27 (10.4%), upper respiratory tract infection 23 (8.9%), and acute otitis media 13 (5.04%).
Clinical characteristics of seropositive children
One-third, 230 (32.38%) of the study participants were categorized to WHO clinical stage 2 and the median CD4 cell count was 644 cells (IQR=288-932). Most, 237 (33.1%) of the under-five children had appropriate developmental status while the less than, 140 (19.20%) of HIV positive children functional status were ambulatory when they start ART (Table 2).
| Variables | Categories | Number | Frequency |
|---|---|---|---|
| WHO clinical stage | Stage 1 | 230 | 32.9 |
| Stage II | 209 | 29.44 | |
| Stage III | 166 | 23.38 | |
| Stage IV | 105 | 14.79 | |
| Adherence of the past 3 months | Good | 353 | 49.72 |
| Faire | 176 | 24.79 | |
| Poor | 181 | 25.49 | |
| Severe Acute Malnutrition (SAM) | 81 | 31.4 | |
| Tuberculosis (TB) | 49 | 18.99 | |
| Pneumosist Carnie Pneumonia (PCP) | 29 | 11.29 | |
| Meningitis | 26 | 10.08 | |
| Opportunistic infection at baseline | AURTI | 23 | 8.91 |
| Acute otitis media | 13 | 5.04 | |
| Skin dermatitis | 3 | 1.18 | |
| Others | 7 | 2.71 | |
| Developmental status for <5 years (N=299) | Appropriate | 179 | 59.88 |
| Delay | 57 | 19.06 | |
| Regression | 21 | 7.03 | |
| Functional status for >=5 years (N=411) | Working | 297 | 72.26 |
| Ambulatory | 79 | 19.22 | |
| Bedridden | 35 | 8.9 | |
| MUAC | <11.5 cm | 278 | 39.15 |
| >=11.5 cm | 432 | 60.85 | |
| Hemoglobin | No anemia | 569 | 80.14 |
| Anemia | 141 | 19.86 | |
| Baseline nutrition status | Normal quintiles (= 2Z score) | 439 | 61.83 |
| Undernutrition (= 2Z score) | 271 | 38.17 | |
| = 100 copies/ml (Undetectable) | 137 | 19.3 | |
| Baseline viral load | 101-1000 copies/ml | 179 | 25.21 |
| >=1000 Copies/Ml | 394 | 55.49 | |
| Baseline CD4 count baseline (cells/mm3) | = 100 cell/mm3 | 32 | 4.51 |
| 101-1000 cell/mm3 | 521 | 73.38 | |
| = 1000 cell/mm3 | 157 | 22.11 | |
| =11 mg/dl | 557 | 78.45 | |
| Hemoglobin | <10.9 mg/dl | 153 | 21.55 |
Table 2: Baseline clinical and immunological characteristics of children on first-line ART at Ethiopia referral hospitals, 2011 to 2018 (N=710).
HAART related characteristics
Of the total 710 participant children, two hundred seventy-five (38.86%) of them at baseline were on AZT-3TC-NVP, and the majority, 353 (49.72%) them had good ART adherence during their last follow-up period. Nearly one in five (24.56%) of the study participants had the experience of regimen change during their ART follow-up time. Among the study participants, 70 (39.19%) had experienced ART side effects during their follow-up period, and mostly with AZT-3TC-NVP regimen, anemia being the commonest 49 (27.68%). Toxicity or side effects were the commonest 38 (51.6%) reason for regimen change. Program shift from D4T to AZT or TDF, and TB incidence shift failure were other reasons. During the follow-up time, the majority 280 (39.75%) of the study participants did not take ART prophylaxis following birth from HIV-infected mothers (PMTCT). Among the children on ART, 411 (57.78%) took Cotrimoxazole and 442 (61.25%) took on their follow-up time Isoniazid prophylaxis. The median ART clinic follow-up period of participants was 41.6 (IQR=24-49) months with a minimum of 6 and maximum of 157 months. Of the total nearly half, 279 (39.39%) of the participants were followed for less than 36 months. The majority, 563(79.30%) of study participants were on ART follow-up at the time of the study, 51 (7.19%) were reported as dead, 37 (5.49%) transfer out, 34 (4.79%) lost from follow up and 25 (3.52%) transferees into adult cohort (Table 3).
Time to first-line ARVI treatment failure
All study participants 710 who were followed for different periods with 1935.24. Person per Year (PYOs) of risk observations, with a minimum of 0.59 years and a maximum of 13.05 years were observed. The median survival time was 42.8 months with 1298.32 person-years follow uptime. Ninety-six, 13.5% (95% CI: 11.18, 16.25) of participants experienced treatment failure. Of these, 47 (48.98%), 21 (21.88%), 17 (17.88%), and 11 (11.46%) of the children have experienced a Virologic, Immunologic, clinical, and mixed (immunological+clinical+virological or two of the three failure), respectively. The Incidence Density Rate (IDR) of first-line ART regimen treatment failure was 4.098 (95% CI: 3.35 to 5.02) per 1000 Person-per Month of observations (Table 4).
Survival function and hazard of failures
Alarmingly, 68/96 (70.2%) of failures were reported after 72 months of the follow-up within 5172.89-person time risk observation. It is also awful that, from 96 children who failed for first-line, only 23 (24%) children were switched to second-line drugs after first-line treatment failure.
However, 614 children didn't experience treatment failure 18049.716 Months of risks of observations. While the incidence rates of treatment failures for children were on WHO stage III and IV was 1.86(95%CI:1.69; 2.04) person per 100 months of risk observation. The cumulative treatment failure at the end of 24, 48, 72, 96 months, and at the end of follow-up were 0.045, 0.29, 0.31, 0.41, and 0.81, respectively. The median follows up and survival time were found to be 41.9 months (95% CI: 38.2, 45.8) and 0.82 (95% CI: 0.7647; 0.8651) respectively (Figures 2-4).
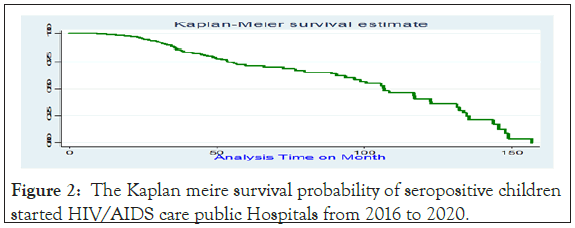
Figure 2: The Kaplan meire survival probability of seropositive children started HIV/AIDS care public Hospitals from 2016 to 2020.
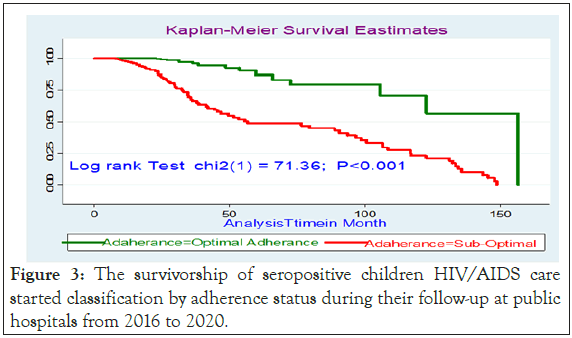
Figure 3: The survivorship of seropositive children HIV/AIDS care started classification by adherence status during their follow-up at public hospitals from 2016 to 2020.
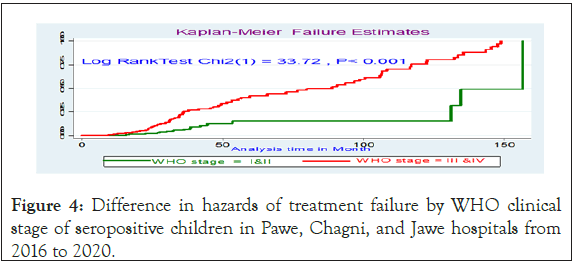
Figure 4: Difference in hazards of treatment failure by WHO clinical stage of seropositive children in Pawe, Chagni, and Jawe hospitals from 2016 to 2020.
Predictors for treatment failure
During bivariable logistic regression age of children, Viral load, WHO clinical stage, CD4 count, residence, parent status, ART regimen, follow up on ART, adherence, IPT and CPT status family size, and baseline MUAC were found significant predictors for treatment failure. After controlling significant confounding of final models, six independent variables were independently associated with treatment failures.
On multivariable analysis, children whose both parents died were 4.3 (AHR: 4.3, 95% CI: 2.17, 7.7) times at a higher risk of treatment failure as compared with those whose both parents were alive. In addition, children who were presented with WHO clinical stage III and IV was 3.5 (AHR: 3.5, 95% CI: 1.8, 7.4) times at a higher risk of experiencing treatment failure than those who were on WHO clinical stage I and II. Moreover, children who had poor ART adherence were 3.27 (AHR: 3.27, 95% CI: 1.54, 4.8) times at a higher risk of experiencing treatment failure as compared with good adherence. The other factor that showed significant association was the duration of ART follow-up; children followed for more than >72 months were 2.28 times (AHR: 2.28, 95% CI: 1.2, 5.2) times increased the risks of experiencing treatment failure as compared being on less follow 30 months. In addition, missing co-trimoxazole preventive therapy was 6.7(AHR-6.7; 95% CI: 3.6, 8.4) times increased the hazards of treatment failures as compared with the counter group. Moreover, children who are on AZT-3TC- NVP treatment regimen were 6.5 (AHR=6.5; 95% CI: 3.2, 18.2) times at a higher risk of treatment failure when compared with those who were on d4t-3TC-NVP. Being on AZT-3TC-EFV were also 2.9 (AHR=2.89, 95% CI: 2.89, 10.1) times at higher risk of treatment failure as compared to those who were on d4t-3TC- NVP. Treatment failure result with d4T-3TC-NVP was associated with; its decreased usage on the research period, after phasing out and is discontinued due to its known long term mitochondrial toxicity and substituted either by AZT or TDF. For anemic patients TDF substitutes AZT and for tuberculosis patients treated with Rifampicin, EFV replaces NVP. Patients on this regimen initially and who stayed till the revised guideline were changed to other regimens. So those patients who were on the d4t regiment were not followed for a long time (Table 5).
Treatment failure is one of the factors which determine the effectiveness of anti-retroviral treatment intervention [35]. While at the end of the study periods, there were 563 (79.3%) on follow up, 4.79% transferee in to adult cohort, 4.85% lost from follow up, 5.5% transfer into another health facility, and 7.18% were died. The overall first-line ART treatment failure of seropositive children was found 13.5% (95% CI: 11.2, 16.3). This finding is higher than with the number of studies previously conducted in Ethiopia such as; in Tigrai regions 11.2% and 8.68, Jimma Hospital (11.5%), Waghimra Zone 11.8%, Bahir Dar, 10.7%, and at four ART center in Addis Ababa 11.7% and Ghana 6.5% [11,16,20,24,36-38]. However, this finding is comparable with reported in Netherlands, Australia and Thailand Research center 14.1%, Zambia 12.3%, 37 Tanzania, 12.3%, Nigeria, 13.7%, South Africa, 13.7%, and Cambodia, 12.9%, but is lower than study findings in Gondar (18.2%), Fiche and Kuyu hospital Oromia (18.9%), Tanzania 25% and (57%), Ghana (29%), Uganda and Mozambique 29%, Senegal 64% [9,16,17,19,29,35,39-43].
The overall incidence rate of first-line ART failure was 4.098(95% CI: 3.35 to 5.02) per 1000 person-per month is lower than as compared with finding in Tigray 8.7 (95% CI 7.10 to 10.60) per 1000 person-month but higher than reported in Amhara region, 2.2(95% CI 1.72 to 2.82) per 1000 person-months [11]. The possible reason might be that they determine failure rate only by immunologic and clinical criteria which could further lengthen the time to detection than viral load criteria. In this study, nearly half of 48/96(49.8%) of participants' children had experienced virologic failures, which is the commonest type of treatment failure followed by immunologic failure and only a small proportion having a mixed failure. Which is consistent with cohort study in Ethiopia and India [22,23,36,44,45]. This variance might be attributed due to differences in cutoff values of viral RNA copies per mL of plasma to be considered as virological failure, the difference among study participants, and duration of follow-up, study design, and variations on the treatment adherence of HAART. The median follows up and median survival time were found to be 41.9 months (95% CI: 38.2, 45.8) and 0.82(95% CI: 0.7647; 0.8651), respectively. Which is consistent with finding in India; 43 months, but lower than report in Tigray 57.7 months, Bahirdare 82 month [11,45,46]. This could be due to the improvements in the therapeutic and diagnostic measures in current visits from an increase in access and variety of more potent drugs nowadays than the past periods; in fact the hazards of treatment failures for children who were on ART ≥ 70 months of follow up were significantly associated with treatment failures. Unlike in this study a long duration of follow up was also reported as having a protective effect on first-line ART failure and Amhara regions [11,47]. Rapid effects like IRIS were very common in the early months after ART start; adherence level of children might increase with time and might be considerably lower in the first few months. Moreover, children whose parents (both) were died were predictors well the treatment failure. This is similar to a study conducted at Bahir dare and Markose Referral Hospitals, and Cambodia [8,19]. In this current study, the existence of poor ART adherence among children on first-line ART was identified as a predictor variable for treatment failure supported binding showed at Amhara regions and Fiche and Kuyu hospital Oromia region [8,35]. HIV positive children without parents or appropriate first-lines have psychological depression or impairment and may not have good adherence which can be resulted in developing ART drug resistance with a run out of immune HIV-positive end up in treatment failure. This implies tracing of a patient from ART clinic need to be strengthened using proper appointment calendar utilization, frequent updating of patient contact address to decreasing lost patients. AZT based regimen was found as a predictor for treatment failure by the current which is similar to a study done in Addis Ababa [20]. In contrary, Nevirapine (NVP)- based regimen was identified as a predictor for virological AZT- based revealed in a study in Ethiopia, Malawi and Botswana, also in Thailand Non-nucleoside reverse transcriptase inhibitors is one of type for Virologic failure [8,17,23,48]. These differences may mirror clinicians’ preference in first-line treatment choice. Country- specific differences may potentially confound the relationships seen between combined ART regimens and treatment failure. On the same way, WHO clinical stage at baseline was a significant predictor of treatment failure, this is consistant with finding in Markose and Bahirdare hospitals, Gondar University Hospital, Fiche and Kuyu hospitals [8,35,47]. This can be explained with children with advanced WHO clinical stage at the initiation of ART could have an increased risk of or concomitant opportunistic infections which further impair the positive effects of the ART on immune cells. Most studies both in our country and others show that low CD4 count at ART initiation and occurrence of severe opportunistic infection (like; Tuberculosis and diarrhea) were significant predictors for first-line ART treatment failure unlike the current study [20,47]. This can be explained with, recently ART started for every HIV positive individual and baseline CD4 is not done routinely for every patient, and severe opportunistic infections may not occur as like as the previous time [13]. Moreover, seropositive children who missed co-cotrimoxazole preventive therapy were significantly associated with treatment failure. Imminently CPT is inexpensive and highly effective in reducing morbidity and mortality causes associated with Opportunistic infection and prevents load of latent TB lung [3,27].
Since the study was a retrospective study, it had its limitation associated with poor documentation. Since the design is secondary, the study was unable to exhaustively explore all predictor’s variables that may affect the treatment failure.
More than sixty-two percent of treatment failures were occurred after 72 months of ART follow up and this incidence of treatment failures was unacceptable as compared with UNAIDS cut-off reference range <10%. Even though efforts are taken to increase ART access, children who had a long time of ART initiation, advanced WHO clinical, non-adherence to ART regimen, and those who missed CPT prophylaxis were at higher hazard of treatment failure.
Data set for this manuscript is with supporting information.
It is not applicable.
All authors declare that they have no competing interest final content of the manuscript.
The authors would like to thanks data collectors of Pawe, Changi, and Jawi Hospitals.
FK, BK, TK, and MG had equally conceived the study, supervised the data collection, project administration, Validation, analysis and wrote the manuscript. The authors commented and edited the draft and approved the final version submission.
Citation: Kebede F, Kebede B, Kebede T, Giza M (2021) Time to First-Line Antiretroviral Treatment Failure and its Predictors for Seropositive Children Treated in Public Hospitals, North West Ethiopia 2021. J Antivir Antiretrovir. S21:001.
Received: 09-Aug-2021 Accepted: 23-Aug-2021 Published: 30-Aug-2021 , DOI: 10.35248/1948-5964.21.s21.001
Copyright: © 2021 Kebede F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.