Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2023)Volume 14, Issue 3
Background: Plane warts are a common viral infection of skin and mucosa causing significant cosmetic concern to the patient, especially if they are multiple and disseminated over the face.
Objective: To compare the effectiveness and safety of low dose oral isotretinoin (20 mg/day fixed) alone against combination with topical tretinoin (0.1%) in gel formulation for the treatment of verruca plana.
Methods: Two verruca plana treatment approaches were compared in a prospective randomized interventional clinical trial.
Two groups of sixty individuals with numerous plane warts were randomly assigned. Group A received oral isotretinoin capsules at a dose of 20 mg daily, whereas Group B received oral isotretinoin at a dose of 20 mg daily in addition to topical tretinoin 0.1% in gel formulation once daily at night. The patients received treatment for 12 weeks. Patients who had a full recovery were monitored for 8 weeks to track the recurrence rate.
Results: Although the difference was not statistically significant, patients receiving combination therapy had more rapid lesion resolution and a higher total percentage of lesion resolution.
Conclusion: Combination treatment leads to faster and complete resolution of the lesions than oral isotretinoin alone. More trials with large sample size needed.
Limitation: Study sample size was small, that was the only limitation.
Retinoid; Isotretinoin; Tretinoin; Verruca plana; Wart
Human Papillomavirus (HPV) infection, particularly HPV-3 and HPV-10, is the primary cause of plane warts. Clinically, it appears as many, slightly elevated, flat-topped papules that are asymptomatic and most frequently affect children and young adults' faces, hands, neck, and legs. They typically range from 1 mm to 5 mm in diameter, are round or polygonal in shape, and are skin-colored or brownish [1]. They may be arranged in a group and in a linear fashion, suggesting that trauma (autoinoculation or pseudokoebenerization) may have had a part in their placement. The widespread distribution of plane warts on the face can significantly deform the patient's appearance and lead to psychological distress.
The available literature search revealed a variety of therapeutic options for treating plane warts, including topical treatments, immunotherapy and destructive operations [1-3]. First-line treatments are usually those which can be used by the patient comfortably at home without necessitating regular hospital visits (for example, topical salicylic acid, lactic acid and retinoic acid, oral zinc and levamisole). Second line treatments include destructive procedures like chemical cautery (by trichloroacetic acid, KOH, phenol), electrofulgration, radiofrequency ablation, cryotherapy, immunotherapy and laser. However these may exacerbate cosmetic problems by causing dyspigmentation (hypo or hyperpigmentation) and scarring. Moreover, both the response rate and length of the treatment are widely variable. The optimum objectives for treating warts should be:
1. Complete clearance of lesions within the shortest time period possible
2. Easy to use
3. Minimal cost
4. Minimal discomfort to patient
5. Producing no cosmetic disfigurement
6. With no relapse
Given that no medication has yet been able to achieve these ideal goals, retinoids seem to be a viable therapeutic option for the treatment of plane warts. Olsen EA, et al. [4], utilised oral retinoids to treat refractory genital warts in 1989 after Gelmetti C, et al. [5], used them to treat several refractory cutaneous wart types in 1987. Later, additional groups reported using different isotretinoin dosages to treat muco-cutaneous human papillomavirus infections and reporting variable percentages of full clearance.
Oral retinoids appear to be a feasible treatment option since they have the potential to influence epidermal development and differentiation, trigger immunomodulation, and reduce the production of the human papillomavirus in infected cells [3,6-8].
Combination of oral and topical retinoid has never been used in the past for treatment of warts, so we decided to use the combination as the authors felt every dermatologist is experienced with the use of oral plus topical retinoid in treating acne vulgaris.
The study was randomized open therapeutic trial carried out at medical college attached tertiary care hospital. The duration of the study was one and half year (from June 2020 to December 2021). Following institutional ethics committee approval, the trial included all patients who provided written informed consent and met the inclusion and exclusion requirements. They were randomized by even odd criteria.
A comprehensive examination was done after taking a complete history on the start, progression, and related systemic disorders of warts. The patient received a thorough explanation of the disease's causes, its course of therapy, and any potential adverse effects. Each patient gave their informed permission. All females in the reproductive age range (>14 years to 60 years) signed a supplemental permission form that described the teratogenic potential of oral isotretinoin. If the patient was a minor (under 18 years old), the guardian's consent was obtained. A lesion count was performed. After receiving the patient's consent, baseline photos were taken, and each patient signed a consent authorising the publication of their facial images.
Along with certain specific tests, standard examinations such as the complete blood count, liver function tests, kidney function tests, random blood sugar, urine examination for routine and microscopy, ECG, and chest x-ray PA view were carried out. At baseline, each patient had an ELISA test for HIV, HBsAg, and anti-HCV, as well as a fasting lipid profile. Throughout the therapy period, tests on the liver function and lipid profile were performed while the patient was fasting. All females in the reproductive age group had baseline urine pregnancy tests done, and they received double contraceptive advice both during the course of the medication and for one month after it was stopped. We were having two treatment plans. Plan 1 was to treat each patient with low dose oral isotretinoin capsules in the dose of 20 mg/day and plan 2 was to treat each patient with low dose isotretinoin capsules in the dose of 20 mg/day with topical gel tretinoin 0.1% once daily application at night. We kept the dose of oral isotretinoin as 20 mg daily in every patient fixed, as this dose was readily available in our set up and this dosing of oral isotretinoin falls under low dose oral isotretinoin (0.5 mg/kg/day), considering the age group of our study population.
Until we discovered 60 eligible patients, we included many verruca plana patients visiting the skin outdoors who met the trial inclusion and exclusion criteria and were prepared to provide written informed permission. As a result, 113 patients in all were screened, and 60 of them were chosen for randomization into two groups of 30 each. The patients were chosen using a thorough randomization approach for both treatment strategies. Every even patient received therapy according to plan 1 and was placed in Group A, while every odd patient received treatment according to plan 2 and was placed in Group B. For the course of the treatment period, patients were followed up every two weeks for a total of 12 weeks to assess their response to therapy and any side effects. Treatment was stopped at 12 weeks irrespective of patient response in all patients (if patients showed complete clearance any time before 12 weeks, then also the treatment was continued). After 12 weeks of treatment, those patients who showed complete clearance were followed up further for 8 weeks thereafter to assess for recurrence.
Each patient was instructed to apply white petrolatum jelly (vaseline) to their lips and moisturizer to their face as needed in order to lower their chance of developing cheilitis and facial dryness. Additionally, patients were told to avoid getting too much sun. Patients receiving topical medication were cautioned against coming into touch with their eyes and mucous membranes, as well as against using abrasive or caustic chemicals concurrently. When a strong response happened, they were instructed to temporarily halt the therapy and see a doctor.
Inclusion criteria
1. Patients >14 years of age of either sex diagnosed with verruca plana.
2. Patients with normal liver function tests and lipid profile.
3. Patients giving written informed consent.
Exclusion criteria
1. Pregnant and Lactating females.
2. Patients suffering from liver, kidney diseases and Sjogren syndrome.
3. Any other contraindication to oral or topical retinoids.
The effectiveness of the treatment was assessed in relation to the decline in lesions. At baseline and after that, every two weeks, lesion counts were done. At every follow-up, patients were assessed clinically and visually. The grading of the response was done as:
1. Complete clearance of lesions i.e., 100%
2. Decrease in number of lesions by 75%-99%
3. Decrease in number of lesions by 50%-74%
4. Decrease in number of lesions by 25%-50 %
5. Decrease in number of lesion by <25%
6. No decrease in number of lesions i.e., 0%.
The definition of the treatment response was:
1. Complete response rate=complete removal of lesions.
2. Partial response rate=decrease in the number of lesions by ≥ 50%.
3. No response=less than 50% or no clearance of lesions.
Statistical analysis
Data presentation was done using a descriptive statistical analysis. To compare data at baseline and after 12 weeks as well as the average lesion score in the two groups, paired and unpaired t-tests were used.
30 patients were included in each group. The mean age of patients of Group A was 25.30+/-7.30 years whereas it was 25.23+/-6.84 years in Group B, both groups' ages were comparable when statistical comparisons were made between them (p=0.971). Similarly, mean number of lesions in Group A was 54.17+/-26.13 while in Group B it was 59.33+/-31.31, which was statistically comparable in both the groups ( p=0.491) (Table 1).
| Characteristics | Group A | Group B |
|---|---|---|
| Age in years (mean) | 25.30+/-7.30 | 25.23+/-6.84 |
| Gender | ||
| Males | 66.67% | 66.67% |
| Females | 33.33% | 33.33% |
| Mean number of lesions | 54.17+/-26.13 | 59.33+/-31.31 |
| Complete response at 12 weeks | 70% | 89.65% |
| Side effects | ||
| Cheilitis | 100% | 100% |
| Dryness of face | 23.33% | 26.66% |
| Telogen effluvium | None | 3.33% |
| Perilesional hypopigmentation | None | 46.66% |
| Raised transaminases | 10% | 6.66% |
Table 1: Summary of characteristics of both the groups.
66.67% of participants in Group A as well as in Group B were males and both the groups were statistically comparable (p=1.00).
The mean number of lesions reduced to 37, 20.77, 4.77 at 4, 8, 12 weeks respectively in Group A from baseline mean of 54.17. Similarly in Group B the mean number of lesions reduced from 59.33 at baseline to 35.4, 18.4, 3.47 at 4, 8, 12 weeks respectively. During 4, 8, and 12 weeks of treatment, there was no significant difference in the mean number of lesions seen between the 2 groups (p>0.05). However the number of lesions was less at all points of observation in Group B as compared to Group A. (Table 2 and Figure 1)
| Group A | Group B | p value Group A vs. Group B | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | p value | Mean | SD | p value | ||
| Baseline | 54.17 | 26.13 | - | 59.33 | 31.31 | - | 0.49 |
| 4 weeks | 37 | 21.26 | p<0.001 | 35.4 | 22.97 | p<0.001 | 0.78 |
| 8 weeks | 20.77 | 14.67 | p<0.001 | 18.21 | 17.61 | p<0.001 | 0.545 |
| 12 weeks | 4.77 | 7.97 | p<0.001 | 2.76 | 8.82 | p<0.001 | 0.331 |
| % decrease 4 weeks | 31.69% | 40.30% | |||||
| % decrease 8 weeks | 61.65% | 69.30% | |||||
| % decrease 12 weeks | 91.19% | 95.30% | |||||
Table 2: Lesions at baseline 4, 8 and 12 weeks in both the groups.
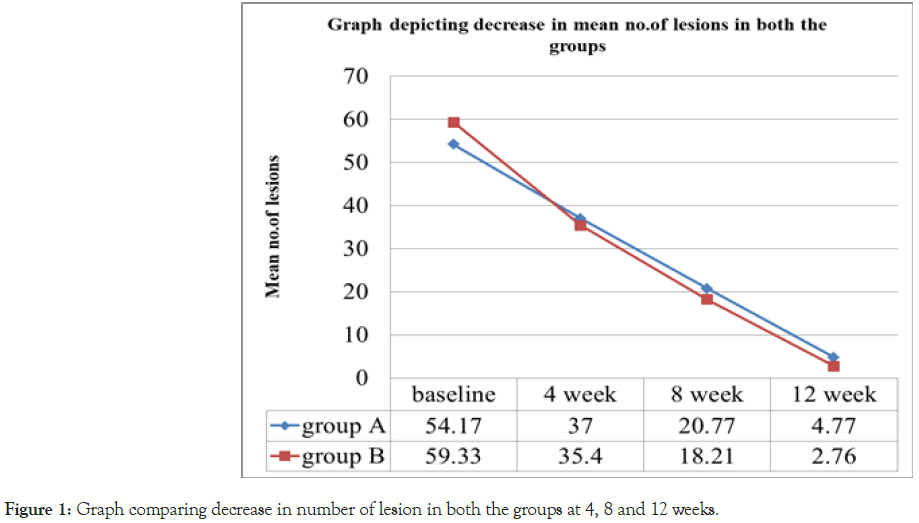
Figure 1: Graph comparing decrease in number of lesion in both the groups at 4, 8 and 12 weeks.
At 8 weeks, in Group A 3.33% of the patients had complete response and 96.66% had partial response while in Group B more number of patients i.e., 27.58% had complete response at 8 weeks while 68.96% had partial response which is statistically significant with a p-value of 0.018.
At 12 weeks of treatment, in Group A, 70% of the patients had complete response and 30% had partial response while in Group B, more number of patients i.e., 89.65% of the patients had complete response after complete treatment of 12 weeks.
However, due to the lower number of participants, this difference was unable to reach statistical significance (p=0.121)
Clinical photographs showing complete clearance has been shown in Figures 2-5.
Figure 2: Lesions at baseline and complete clearance at the end of 12 weeks in a Group A patient.
Figure 3: Lesions at baseline and complete clearance at the end of 12 weeks in a Group B patient.
Figure 4: Perilesional hypopigmentation observed with topical tretnoin gel in a Group B at 8 weeks of treatment and clearance of lesion at the end of treatment.
Figure 5: Lesions at baseline and complete clearance of lesions at 8 weeks in a Group B patient.
100% of the patients had cheilitis in both Groups A and B. 23.33% of patients in Group A while 26.66% of patients in Group B had dryness of face. 23.33% of patients in Group B had local irritation due to topical tretinoin. 1 Patient in Group B i.e., 3.33% had telogen effluvium. Perilesional hypopigmentation was seen in 14 (46.66%) patients in Group B due to topical tretinoin, which was observed at 4 weeks and continued till 12 weeks during treatment, however it subsided in all patients at 8 weeks of follow up visits. Moreover, laboratory investigations showed elevated SGOT and SGPT in overall 5 (3 in Group A and 2 in Group B) participants, however this was below twice the upper normal limit at all times and returned to normal during the follow up period. None of the patients experienced elevation in lipid profile.
Relapse was not seen at 8 weeks follow up in any of the patient who completely cleared at 12 weeks treatment in both the groups. Only 1 patient in Group B was lost to follow-up during treatment at 8 weeks.
Plane warts are a frequently occurring dermatological disease, treatment of which is challenging due to the occurrence of multiple lesions over exposed parts (most frequently over face) causing significant mental trauma in patients. Recalcitrant nature of the disease and frequent recurrences adds to this stress. There is a vast array of wart treatments available, such as topical ones (like retinoic acid, trichloroacetic acid, phenol, imiquimod and 5-fluorouracil). Interventional therapies like electrofulgration, autoinoculation, cryotherapy and immunotherapy with variable results are also available. Above modalities are not satisfying because they are painful, inconvenient, can cause scarring and pigmentary changes (hypopigmentation or hyperpigmentation) which is worrisome on the face. Cost of the procedure as well as the recurrence rate with the above mentioned modalities is high leading to unsatisfaction among the patients.
Structurally and biologically, retinoids are similar to vitamin A. These epidermal alterations combined result in clinical desquamation and peeling and may potentially suppress the replication of HPV by immunomodulation. They influence keratinocyte differentiation and proliferation. Retinoids appear to be promising first line of treatment in patients with disseminated disease.
On detailed literature review some studies were available which have used either oral isotretinoin (high as well as low doses) or topical tretinoin in varying concentrations. None of the study has used combination of oral and topical retinoid.
According to Kaur GJ, et al. [3], 38% of patients receiving topical isotretinoin (0.05%) showed full response, compared to 69% of individuals receiving low dosage oral isotretinoin (0.5 mg/kg/ day) who had complete remission. Patients received treatment for three months or until all lesions were disappeared. The patients were all older than 20. They discovered that individuals whose therapy was discontinued earlier because to early clearance had recurrence more frequently. So we thought of giving regular therapy for 3 months in order to reduce the chances of relapse. Results of our study showed similar efficacy, but at the same time there was no relapse in completely cleared patients.
A series of children were treated for warts with 0.05% tretinoin cream in a research by Kubeyinje EP, and the warts cleared up 84.6% of the time, compared to 32% of spontaneous clearance in the control group [9]. This study differed from our study as here the patient population was children and concentration of topical tretinoin was 0.05%, while in our study all patients were adults and elderly and concentration of topical tretinoin was 0.1%. Higher results with only topical tretinoin in children may be due to higher tretinoin penetration in thin skin of pediatric age group. But this study enforced us to use topical tretinoin in combination with oral isotretinoin. Topical isotretinoin gel was employed in a trial by Fadheel BM, et al. [10], which treated plane warts in patients of all ages. The concentration of the gel was not indicated by the author. This study showed 53.7% clearance which was much less than the results shown by Kubeyinje EP, [9]. The less response in this study may be due to inclusion of adult patients, whose skin is thick enough and there might be less penetration of topical tretinoin.
Verruca plana were treated with low-dose oral isotretinoin alone in a trial by Al-Hamamy HR, et al. [11], 73.07% of patients had complete clearance, which is similarly consistent to our study. However in their study, the age group included varied from 5 years to 35 years while it was more than 14 years in our study, as we considered risk of premature epiphyseal closure by oral isotretinoin before this age group [12]. The treatment in above study was given in the dose of 0.5 mg/kg/day for 2 months while in our study it was given for 12 weeks and in their study 15 out of 19 patients were still completely free from verruca plana after four month follow up, while in our study none had relapse.
Olguin Garcia MG, et al. [13], investigated the effects of oral isotretinoin (30 mg/day) for 12 weeks in 16 patients with resistant facial verruca plana and found that 100% persons in the oral isotretinoin group had full clearance, unlike the placebo group, which showed no improvement. The patients' median age was 25, which is close to the findings of our study. The authors, however, did not monitor the patients for relapses.
In their meta-analysis, Yang TH, et al. [14], found that oral isotretinoin is more efficacious than placebo for treating resistant HPV infection of skin and mucosa. Patients with plane warts and condyloma acuminatum both experienced full responses to oral isotretinoin at rates of 64.9 and 76.6%, respectively. Their investigation found that the combined complete response rates to isotretinoin at high and low doses were 63.7 and 71.6%, respectively. There were no head-tohead comparisons to evaluate in the literature. These findings suggest that low-dose isotretinoin (0.5 mg/kg/day) and highdose isotretinoin (1 mg/kg/day) are equally effective in treating mucocutaneous Human Papillomavirus (HPV) infections. This meta-analysis also provoked us to use low dose oral isotretinoin.
In a research by Gelmetti C, et al. [5], 80% of kids who received oral etretinate tablet once a day for 90 days exhibited full regression, while the other kids only demonstrated partial regression.
After two months of treatment with oral acitretin 1 mg/kg/day, Choi YL, et al. [15], fully eliminated a 25 years adult male with multiple verrucae on bilateral hands.
The hands and feet of a 33-year-old male with HIV who had numerous large hyperkeratotic cauliflower like warts were treated with acitretin 25 mg/day by Simone CD, et al. [16]. Within two weeks, the lesions began to shrink, and by two months, they had significantly improved. After six months of follow-up, there were no indications of recurrence [17]. Various other trials have also shown the efficacy of systemic retinoids not only in flat warts but also in other variants of warts [18-20].
As there were multiple studies to show isolated use of either low dose oral or topical retinoids and as we have seen good responses of combining low dose oral isotretinoin with topical retinoid in acne patients, we thought of combining this regime for verruca plana patients.
In our study at 8 weeks, in Group B (combination therapy) 27.65% patients had complete clearance of lesions whereas only 3.33% had complete clearance in patients receiving oral isotretinoin alone showing that the response is faster with combination therapy. The percent of complete clearance of lesions increased to 89.65% of patients in Group B while was 70% in Group A at the end of treatment i.e., 12 weeks. The percentage clearance was much more in Group B, but this was statistically not significant, this probably may be due to the small number of sample size in both the groups.
Additionally, neither group's side effect profile was particularly severe. The use of topical and oral retinoids in our study prevented post-inflammatory hyperpigmentation and scarring, which were not seen with the use of conventional firstline therapies for plane warts like cryotherapy and topical trichloroacetic acid. This highlights the safety and efficacy of retinoids in treating plane warts while also preventing cosmetic disfigurement. Minor transaminase increase, cheilitis, and mild hypopigmentation were seen in individuals using topical tretinoin, although none of these side effects were severe enough to warrant stopping the medication. Also with continuation of the treatment for 12 weeks, there was no relapse of the warts in the patients achieving complete clearance at 12 weeks when followed up for a further period of 8 weeks.
Our study highlights that systemic low dose oral isotretinoin when combined with topical tretinoin 0.1% can be superior to low dose oral isotretinoin alone in terms of faster resolution of lesions in addition to complete clearance with minimal side effect profile. We recommend treatment to be continued for at least three months to reduce chances of relapse.
The number of patients was more from those in published literature but still the authors feel that it was small to draw a firm conclusion.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Randhawa NK, Kumar S, Agarwal US, Agarwal P, Bansal A, Meena S (2023) To Evaluate Efficacy and Safety of Low Dose Oral Isotretinoin (20 mg/ Day) Alone Versus Combination of Low Dose Oral Isotretinoin (20 mg/Day) Plus Topical Tretinoin (0.1%) in Gel Formulation in the Treatment of Verruca Plana. J Clin Exp Dermatol Res.14:635.
Received: 20-Feb-2023, Manuscript No. JCEDR-23-21843; Editor assigned: 23-Feb-2023, Pre QC No. JCEDR-23-21843 (PQ); Reviewed: 09-Mar-2023, QC No. JCEDR-23-21843; Revised: 16-Mar-2023, Manuscript No. JCEDR-23-21843 (R); Published: 23-Mar-2023 , DOI: 10.35841/2155-9554.23.14.635
Copyright: © 2023 Randhawa NK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.