Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Research Article - (2024)Volume 11, Issue 4
Monitoring of general practice antibiotic prescribing is important to allow concordance with prescribing guidelines to be assessed. Antibiotics have dramatically changed the prognoses of patients with severe infectious diseases over the past 50 years. However, the emergence and dissemination of resistant organisms has endangered the effectiveness of antibiotics. One possible approach to the resistance problem is the appropriate use of antibiotic drugs for preventing and treating infections. This study describes how the volume and appropriateness of antibiotic use in hospitals vary among different hospitals of Lakki Marwat, Bannu and D.I. Khan. Changing hospital antibiotic use is a challenge of formidable complexity. On each level, many determinants play a part, so that the measures or strategies undertaken to improve antibiotic use need to be equally diverse. Although various strategies for improving antibiotic use are available, a programme with activities at all three levels is needed for hospitals. The result of our study shows significant result showing multiple use of antibiotics along with multi drug therapy that will definitely leads to the resistance of antibiotics in patients. Our study also tells us about the use of those antibiotics that should be given in last stages are prescribing to the mild ill patients that will leads to ineffectiveness of the antibiotics. Some patients are given the antibiotics that do not need antibiotics at all. We can conclude from our study that antimicrobial resistance can recognize trends in resistance patterns and novel resistances. Pharmacist should pay an active role at all levels especially in hospitals to improve the standard of life, to stop the malpractice of drugs specially antibiotics, to avoid the drug and food complex formed with antibiotics and to prevent the patient from resistant to the antibiotics.
Antibiotcs; Prescribe; Pharmacy; Medicine
Rational use of drug
Rational use of drugs is based on use of right drug, right dosage at right cost which is well reflected in the World Health Organization (WHO) definition: Rational use of drugs requires that patients receive medications appropriate to their clinical needs, in doses that meet their own Individual requirements for an adequate period of time, at the lowest cost to them and their community.
Antimicrobial drug resistance
Antimicrobial drug resistance refers to non-responsiveness of micro-organisms to an antimicrobial agent. It can be intrinsic or acquired.
Rational prescribing
Prescribing right drug in adequate dose for the sufficient duration and appropriate to the clinical needs of the patient at lowest cost.
Poly pharmacy: Poly-pharmacy is the concurrent use of multiple medications.
It can be associated with the prescription and use of too many or unnecessary medicines at dosages or frequencies higher than therapeutically essential.
FDCs: It abbreviated form of fixed dose drug combinations.
Patient demography: It represents patients’ Information like age, Gender, disease state etc.
Aims and objectives
The purpose of this study was to assess the prescribing behavior of antimicrobials, while using WHO guidelines for prescribing antimicrobials. The aims and objectives were to check:
• Patient demography
• Prescriber information
• Poly pharmacy
• Drug-Drug Interactions
• Contra indications
Diseases of bacterial origin are a major cause of morbidity and mortality in low-income countries. Many of these conditions can be prevented with improved personal hygiene, immunization, and environmental sanitation, but antibacterial drugs are still the main therapy for many of them. This key role of antibiotics has led to high levels of consumption and spending for this category of drugs. In low-income countries, antibiotics are available to the public from a variety of sources, including hospitals and pharmacies, licensed medicine stalls and drugstores, and roadside stalls and hawkers. They can be purchased without a prescription in most low-income countries, even when this practice is illegal. This widespread availability has led to inappropriate use by patients and health care providers, and a steady increase in drug resistance [5].
The concept of rational use of drug is new in developing countries, though several steps have been taken in the recent past towards ensuring rational drug use. Among the various measures, the development and revision of national essential drug list, development of national formulary, amending pharmacy act and opening drug information centres are vital.2 on one side the members of the healthcare team (physician, nurse, pharmacist) are needed to practice rational drug therapy in order to ensure patient safety but cannot be implemented without prior patient knowledge regarding medication and their use.
Currently, accurate prescribing decisions, appropriate treatment, and Rational Use of Drugs (RUD) are major concerns among healthcare services. The results obtained after auditing prescriptions indicate that majority of the prescribers do not adhere to the ideal pattern of the prescription writing, and these prescriptions are not explicit in their contents. Replacement of Rx sign with the word ‘Advice’ in large number of prescriptions is indicative of changing pattern of the prescriptions. Overprescribing indicates the increasing tendency of poly-pharmacy. Overuse of antibiotics and injections is also commonly observed.
The trend of the polypharmacy may be due to the patient’s expectations and demand of quick relief, the incorrect diagnosis, and the influence of the lucrative promotional programmes of the drug companies. More than 50% of the inappropriate therapy shows that prescribers are not up to date with the progress in medical field and should be more responsible.
Drug use is influenced by cultural preferences and beliefs. Antibiotics may even be culturally reinterpreted, or ‘indigenized’. People draw these originally ‘foreign’ objects into their own world by clothing them with explanations and meanings from their own culture. Antibiotics do not escape this reality. However, despite these ethnographic accounts of antibiotic use, the factors that determine poor antibiotic usage practices are not yet well understood. Much literature on drug use has been generated in recent years, but most documents go little further than the mere quantitative aspects of antibiotic use (for example, rates, percentages and costs). Similarly, recommendations for improvement go little further than calling for a ‘change of attitude’, training activities to improve inappropriate practices, and implementing national drug policies.
The World Health Organization recently released its global strategy for the containment of antimicrobial resistance (WHO, 2001). This document reflects the consensus of experts from industrialized and non-industrialized countries on how to address the growing problem of resistance to antimicrobials. Resistance to first-line drugs is said to ‘cost money, livelihoods, and lives and threatens to undermine the effectiveness of health delivery programme’, or even pose ‘a threat to global stability and national security’. Non-industrialized countries, home to the majority of the world's population, have an important role in the emergence of global resistance. The Strategy proposes a variety of measures, including reducing the burden of disease and improving access to appropriate antimicrobials. But, as the WHO document asserts, ‘antimicrobial use is influenced by an interplay of the knowledge, expectations and interactions of prescribers and patients, economic incentives, characteristics of the health system(s), and the regulatory environment’. Hence, reducing global antibiotic resistance is as much an issue of behavioral change, as changing health systems and development of new antibiotics [2]. Sadly, this is where the strategy gives much less guidance.
The advent of antibiotics, which are some of the most successful drugs in medicine, dramatically altered the prognoses of patients with bacterial infections. Their power in both therapy and prophylaxis was so convincing that many older antibiotics have never undergone controlled clinical trials. However, the excessive and indiscriminate use of these so-called miracle drugs in both human and veterinary practices has led to the emergence and dissemination of resistant organisms that endanger their efficacy. Major problems are encountered for an increasing number of pathogens, including Staphylococcus aureus, Streptococcus pneumonia, and Clostridium difficile. For example, the use of almost any antimicrobial drug has the potential to induce the onset of C. difficile infection.
As a consequence, C. difficile infection is the leading cause of hospital-acquired infections in most high-income countries, and resistance is an increasing problem.
Resistant pathogens cause infections associated with greater mortality and morbidity. Antibiotic resistance has a substantial economic impact because of the need for more expensive second-line drugs and longer hospital stays associated with therapy failure. Some studies suggest a relation between resistance rates and the volume of antibiotic use. In addition, modelling studies show the value of infection-control practices and restricted use of antibiotics to control methicillin-resistant S. aureus in hospitals. Similarly the quality of both infectioncontrol practices and antibiotic use plays a part in the incidence of C. difficile infection [6]. One way of tackling resistance is to use antibiotics appropriately to prevent and treat infections.
Antimicrobials, when appropriately targeted to a susceptible pathogen, improve outcomes for patients. However, use and overuse at the population level is associated with the emergence of bacterial resistance. Behaviors that promote resistance in one individual or population can have wider consequences for the global community. These behaviors extend beyond human antimicrobial use to areas such as antimicrobial use in livestock production. Although non-prescription antimicrobial use is typically studied within a single country, the effect of widespread antimicrobial overuse is likely to be felt worldwide.
Reason
The reasons that are most common that leads to the antibiotic resistance include:
In-appropriate antibiotic use in hospitals
In many countries, a big chunk of the total drug budget is allocated to antimicrobials and they are often the single largest group of medicines purchased in developing countries. Studies have also shown that about one third to half of all hospitalized patients receive an antibiotic, which accounts for more than 30% of hospital budgets in many hospitals [8]. Increasing drug cost is a burden to many healthcare delivery systems in both developed and developing countries.
Appropriate antibiotic use in hospitals entails finding a middle road between their potent ability to reduce the mortality and morbidity of patients with infectious diseases and their potentially hazardous effects (i.e, serious adverse events, drug interactions, and induction of resistant strains). Unnecessary use of antimicrobial agents, and use of the newest, broad-spectrum antibiotics when narrow-spectrum and older agents would suffice can lead to increases in resistance, harm patients, and increase treatment costs. Conversely, unjustified therapy with narrow-spectrum agents that ineffectively treats the causative pathogen can also be detrimental to the patient. The role of antibiotic stewardship programme is to strike a balance between the potent ability of antibiotics for individual patients and their potentially hazardous effects. Initiatives to support appropriate antibiotic use are relevant because of its effects on bacterial resistance, clinical outcome and costs.
By definition, guidelines are documents that include a set of statements about appropriate care. In the field of infectious diseases, guidelines are developed both from a perspective of infection control and management of infectious diseases. These guidelines are developed and disseminated by, for example, the infectious diseases society of America, the US centers for disease control and prevention, the British society for antimicrobial chemotherapy, and the UK national institute for health and clinical excellence. Studies have shown that 30%-40% of patients do not receive care based on available scientific evidence according to guidelines, and 20%-25% of the health care provided is unnecessary [7]. The findings for antibiotic care are similar, and assessments have found that up to 50% of hospital antibiotic use is inappropriate. Thus, there is ample room for improvement. But how can we improve it?
There is limited knowledge of the key determinants of Antimicrobial Prescribing Behavior (APB) in hospitals. An understanding historically, the ability to prescribe antibiotics changed the therapeutic power of physicians in an unprecedented fashion. Although antibiotics have lost much of their glamour due to the increasing antimicrobial resistance of microorganisms, this sense of power probably still underlies antibiotic prescription. Thus, changing prescribing habits in hospitals can be a challenge. Unfortunately, a rather naive approach to changing professional behavior is often used to meet this challenge. Presenting information on innovations (scientific papers, reviews, guidelines, care bundles, antibiotic booklets, and information about inappropriate care) to medical professionals is assumed to ensure that care will be optimized accordingly. It is also assumed that professionals have the time, motivation, skills, and resources to apply this new knowledge and to change clinical practice. Research into changes of professional behavior shows varying and often only modest improvement by this approach [9]. Most innovations require further efforts, or so-called implementation strategies.
Different strategies exist to implement guidelines and other innovations, including educational meetings, feedback, reminders, financial incentives, and revision of professional roles. Many studies have assessed these strategies for improving patients' care. Reviews, and even reviews of reviews, have summarised them. They conclude that there is no superior strategy or so-called magic bullet that works for all innovations in all circumstances. The challenge lies in building a strategy on the careful assessment of obstacles and on a coherent theoretical base [3].
Antimicrobial resistance is a global issue. Resistance genes spread throughout the world, as shown by the global spread of CTX-M Extended Spectrum β-Lactamase (ESBL), NDM-1, or Klebsiella pneumoniae Carbapenemase (KPC) producing Enterobacteriaceae. Travelers might acquire resistant bacteria that can be transmitted in their home countries. Transmission between developed and developing countries is presumably twoway and is rarely identified in developing countries because of inadequate surveillance [4].
Self-medication
Self-medication can be defined as the use of drugs to treat selfdiagnosed disorders or symptoms, or the intermittent or continued use of a prescribed drug for chronic or recurrent disease or symptoms. It is usually selected by consumers for symptoms that they regard as troublesome to require drug therapy but not to justify the consultation of a prescriber. In developing countries, most illnesses are treated by selfmedication [10]. A major shortfall of self-medication is the lack of clinical evaluation of the condition by a trained medical professional, which could result in missed diagnosis and delays in appropriate treatments.
A major problem with self-medication with antimicrobials is the emergence of human pathogens resistance. Antimicrobials resistance is a current problem world-wide particularly in developing countries, where antibiotics are often available without a prescription. Resistance to antimalarial drugs has also been reported in many third world countries. Reasons for this resistance include the irrational use of antimalarial, including their indiscriminate non-prescription use [1].
Medication adherence
Medication adherence can be defined as the extent to which a person’s medication-taking behavior coincides with medical advice.
• Complete medication adherence occurs when the patient
follows the physician’s instructions completely. Possible signs
of no adherence include failure to fill prescriptions, loss to
follow-up, and the absence of serum drug concentrations on
laboratory testing. Partially compliant patients take incorrect
doses of their drugs regularly or correct doses more or less often
than prescribed.
• Studies have reported medication adherence rates in the
elderly that range from 26% to 59%”.
Medication adherence is likely to be increasingly important in the management of disease in this population. As average life expectancy increases, so will the incidence of chronic diseases and the number of patients receiving long-term drug therapy.
Majority of the patients shows noncompliance to the therapy which may be due to the following reason:
• Economic consequences
• Side effects of medication
• Lack of education
• Psychological thinking of patients
• No pharmacist role
• Length of disease in case of T.B etc.
Irrational prescription
Rational use of medicines has been defined by the World Health Organization (WHO) in 1985 as: “Rational use of drugs requires that patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements for an adequate period of time, and at the lowest cost to them and their community”.
Irrational prescribing is a global problem. Bad prescribing habits lead to ineffective and unsafe treatment, exacerbation or prolongation of illness, distress and harm to the patient, and higher costs. Irrational prescribing patterns are perpetuated through patient pressure, bad example of colleagues, and highpowered salesmanship by drug company representatives. In teaching hospitals, new graduates will copy them, completing the vicious circle. Changing existing prescribing habits becomes very difficult [11].
This implies that, irrational use of medicines includes all of the practices that make the mentioned processes of appropriate medicine prescribing not fulfilled. In other words, irrational prescribing can be described as the medically inappropriate and economically ineffective use of pharmaceuticals. It is a commonly observed practice that occurs in both developed and developing countries, with enormous costs from the perspectives of the scarce resources and adverse clinical consequences. It may occur in different forms; misuse, overuse, polypharmacy, adverse drug events or drug-drug interactions. Medication abuse describes a situation, when someone uses medicines very frequently that it becomes harmful, while medication misuse is simply, when someone doesn't use the medication correctly. Using many medicine concomitantly is known as polypharmacy, which is defined as using 2-5 medicines simultaneously. The average number of drug per prescription, the percentage of prescription of antibiotics and injectable drugs are some of the indicators to evaluate the regional status of prescription and medicine practice. According to the reports the average number of drugs per prescription in 12 developing countries is 2.2-3.8 [12]. This average is 1.3-2.2 in developed countries.
Problem caused by antibiotics
• Indiscriminate prescribing of intravenous third generation
antibiotics which should be left as reserve drugs for critically
ill patients.
• Irrational use of third generation cephalosporins leads to
selection of resistant bacterial strains. With little choices of
other reserve antibiotics, serious infections would be very
difficult to manage.
• Overuse of third generation cephalosporins leads to
emergence of resistant bacteria such as methicilin resistant
Staphylococcus aureus.
• The use of intravenous medicines in an outpatient setting
without appropriate community nursing support leads to risks
such as indwelling line infections.
• The reliance on intravenous medicines instead of oral
medicines is defined by the WHO as irrational prescribing.
Solutions:
• Hospitals should have a functioning microbiology laboratory
that produces sensitivity data on prevalent organisms. This
would provide clinicians with good data on antibiotics to be
used as empirical therapy and eliminate the need to resort to
third generation cephalosporins.
• The prescribing of third generation cephalosporins should be
subject to strict guidelines and only allowed after confirming
sensitivity data from microbiology.
• An antibiotic policy should be developed by the pharmacy and
therapeutics committee in liaison with microbiology
laboratory.
• The policy should restrict the prescribing of third generation
cephalosporins to senior prescribers and for a limited period
of time until definitive sensitivities have been confirmed.
Wrong diagnosis
As we know that wrong diagnosis can ultimately leads to wrong prescription of drugs so, it’s very important to have right diagnosis of a patient otherwise patient will face severe consequences that may cause the death of the patient. Wrong diagnosis may be due to.
Physician mistakes: The commonly mistakes that occur from physicians that leads to wrong diagnosis include:
• Over-busy doctors and other medical staff.
• Over-tired doctors from excessive time schedules.
• Slow adoption of new technologies.
• Simple human mistakes: Everyone makes them, even the best
doctors.
• Doctors who are drunk or on illicit drugs.
• Poor handwriting: Can lead to errors in filling prescriptions or
wrong hospital medications or tests.
• Poor dosage instructions: difficult to read numbers, such as
zeroes and decimal points, can lead to wrong dosages.
Patients mistakes: Some of the common patients mistakes can also leads to wrong diagnosis of disease. Some of these mistakes that patient done are given:
• Failure to report symptoms: Some patients do not tell the
doctor about all their symptoms for various reasons
(embarrassment, thinking it will be irrelevant, the doctor
didn't specifically ask about it, etc.).
• Delay in reporting symptoms: This is a very common human
tendency, a form of denial that something is wrong.
• Failure to report other medications they are on, either
prescription or over-the-counter medications.
• Failure to report other alternative medicines they are taking.
• Non-compliance with treatment plan or medications: Overlooked
medications, financial troubles, laziness, etc.
• Dishonesty of patients: Certain hypochondriac and factitious
syndromes, desire to obtain restricted drugs, malingering,
insurance fraud, getting time off work, etc.
• Fear of legal issues: e.g. failure to admit to taking illicit drugs
• Fear of social issues: e.g. failure to admit to lifestyle or social
habits.
• Fear of doctor's scolding: e.g. failure to admit to not following
treatments.
• Failure to read medication labels and instructions fully.
Pharmacist mistakes: The dispensing of medications at the pharmacy can be the source of various mistakes.
• Wrong medication dispensed.
• Similarly labeled or packaged medications wrongly given.
• Similarly named medications confused (by doctor or
pharmacist).
• Wrong dosage dispensed.
• Failure to communicate instructions on taking medication.
Study setting
Lakki Marwat, Bannu, and D.I.Khan are the three cities of Khyber Pakhtunkhwa province, Pakistan. These three cities are located at south of KPK. They are not properly developed in accordance with the health care facilities. So there occurs lot of problems in the use of drugs especially antibiotics.
Study design
The study was generally cross-sectional, prescription review study. The study was designed to describe the prescribing behavior of prescribers, especially of anti-microbials, in health care centers of lakki Marwat, Bannu and D.I.Khan.
Data collection and analysis
The prescriptions were collected from OPD of hospitals and also from the medical stores in these cities i.e Lakki Marwat, Bannu and D.I. Khan. The hospitals from which the data were collected include DHQ hospital Bannu, DHQ hospital Lakki marwat and DHQ hospital D.I. Khan. Data of 200 prescriptions were evaluated in the aforementioned 3 cities, 65 prescriptions from Bannu, 60 from Lakki Marwat and 75 from D.I. Khan that include rates, percentages or indicators for antibiotic prescribing, dispensing or use. We found the antibacterial prescribing for 10 leading indications including upper Respiratory Tract Infection (RTI), lower RTI, sore throat, urinary tract infection, otitis media, conjunctivitis, malaria, vague skin infections without a clear diagnosis, sinusitis and otitis. The patients selected for our study includes those that have some microbial consequences, other microbial consequences or physician has prescribed anti-microbial drugs to the patient [13]. The patients include both OPD patients of hospitals and those visited to private clinics. Assessment of the adequacy of antibiotic use was based on the concept of Rational use of drugs as pro-posed by the world health organization.
These were then analyzed for the above mentioned objectives by using:
• British National Formulary (BNF)
• E-drug index
• Medscape application
• Pharma guide
• Internet source
200 prescriptions from all the three cities were obtained, in which 60 prescriptions were obtained from Lakki Marwat with 42 from DHQ and 18 from Clinical settings, 65 from Bannu with 48 from DHQ and 17 from private clinics, similarly 75 from D.I.K with 55 from DHQ and 20 from clinical settings as shown in Figure 1 and Table 1. These were then analyzed for following various parameters.
• Patient demographic information
• Prescriber information
• No’ of drugs per Rx (encounter)
• No’ of antibiotics/antimicrobials including FDCs per Rx
• No’ of FDCs per Rx (encounter)
• Availability of information for antimicrobials use
• Drug-drug interactions
• Contra-indications
When prescriptions were analyzed for demography of patients it shows that Govt; hospitals has no such prescription which have any demography of patient while 11 prescriptions of private hospitals from Lakki Marwat, 07 from Bannu and 12 from D.I.K have the information about the corresponding patients. This is the 1st step toward irrational prescribing, dispensing and use of drugs as represented (Figure 2).
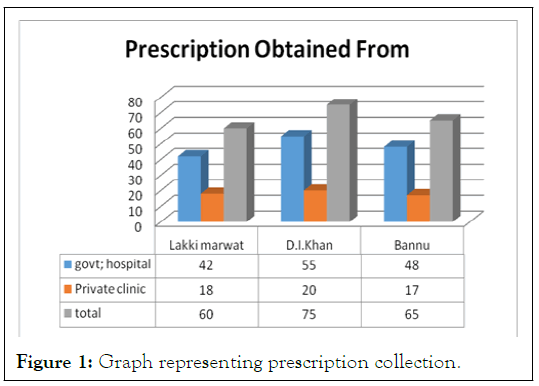
Figure 1: Graph representing prescription collection.
| City | Government hospitals | Private hospitals | ||
|---|---|---|---|---|
| Available | Not available | Available | Not available | |
| Lakki Marwat | 0 | 42 | 11 | 7 |
| Bannu | 0 | 48 | 7 | 10 |
| D.I. Khan | 0 | 55 | 12 | 8 |
| Total | 0 | 145 | 30 | 25 |
Table 1: Showing patients’ demography.
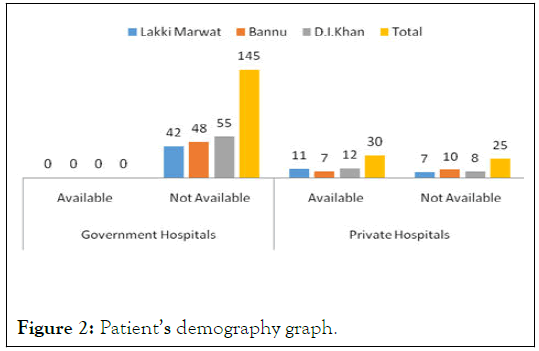
Figure 2: Patient’s demography graph.
Similarly no’ of drugs per encounter were checked and analyzed. We found that 11% Rx were with only 2 drugs/Rx, 38.5% prescriptions were with 3 drugs/Rx, 29.5% with 4 drugs/Rx, 16% with 5 drugs/Rx and 5% prescriptions were with more than 5 drugs per encounter. While the average number of drugs per prescription is 3.67 (Table 2). Which is much higher than the proposed as this average is 1.3-2.2 in developed countries. This will leads to poly-pharmacy, which has potential towards the drug-drug interactions, and in turn to Irrational Prescribing (Figure 3).
| No’ of drugs/Rx | No’ of Rx | % age of Rx |
|---|---|---|
| 2 | 22 | 11 |
| 3 | 77 | 38.5 |
| 4 | 59 | 29.5 |
| 5 | 32 | 16 |
| Many | 10 | 5 |
| Total | 200 |
Table 2: Showing no. of drugs prescribed per prescription.
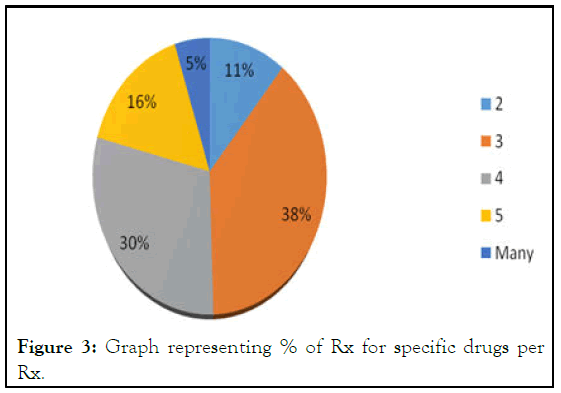
Figure 3: Graph representing % of Rx for specific drugs per Rx.
While the no’ of antimicrobials along with FDCs per prescription disclosed that 86 prescriptions (43%) have 1 Ab/Rx, 73 encounters (36%) are with 2 Ab/Rx, 34 prescriptions (17%) were with 3 Ab/Rx, while 7 encounters (4%) have 4 Ab/Rx respectively (Tables 3 and 4). This indicates, 21% prescriptions have multiple antimicrobial agents, which shows high degree of misuse of Antibiotics and this also leads to the microbial Resistance toward the antibiotics [14]. FDCs were also prescribed like Ampiclox, Augmentin and Artem with 71 prescriptions have only 1 FDCs/Rx, while 5 prescriptions with 2 FDCs/Rx. Overall FDCs prescription rate 38% (Figure 4).
| No’ of Ab+FDCs/Rx | No’ of Rx | no’ of Ab+FDCs |
|---|---|---|
| 1 | 86 | 86 |
| 2 | 73 | 146 |
| 3 | 34 | 102 |
| 4 | 7 | 28 |
| Total | 200 | 362 |
Table 3: Showing no. of antimicrobials per prescription.
| No’ of FDCs/Rx | No’ of Rx | No; of FDCs |
|---|---|---|
| 0 | 124 | 0 |
| 1 | 71 | 71 |
| 2 | 5 | 10 |
| Total | 200 | 81 |
Table 4: Total no. of FDCs’ prescribed=81.
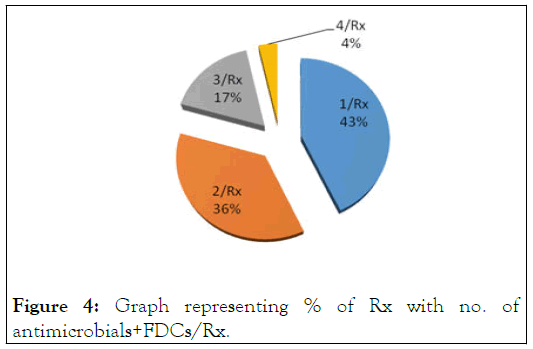
Figure 4: Graph representing % of Rx with no. of antimicrobials+FDCs/Rx.
While the prescribing rate of antibiotics were with the highest frequency of 22.93% is Metronidazole, Arthemeter/ lumefantrine (FDCs) with frequency of 15.47% lies at the 2nd position, while ciprofloxacin with 14.36% has 3rd prescribing rate. Levofloxacin has prescribing rate of 10.22%, azithromycin with 8.29%, cephradine has 2.49%, cefaclor has 0.55%, cefixime 1.66%, ceftriaxone 3.31%, clarithromycin has 3.87%, amoxicillin 1.66%, vancomycin 4.97%, moxifloxacin 3.31%, while that of FDCs, ampicillin+cloxacillin has prescribing rate of 3.04% and amoxicillin+clavolanic acid has 3.83% of prescribing rate.
This shows that prescribing behavior of broad spectrum antimicrobial is much higher than that of narrow spectrum antibiotics. Thus leading to the in-efficacy of narrow spectrum antibiotics and increases the microbial resistance toward antibiotics (Table 5). Some prescribers have prescribed these antibiotics without any formal information to the users, which may cause over dosage or under dosage of these antimicrobials leading to toxicity or in-effectiveness respectively. The drugdrug interactions were found with the rate of 28% minor/ significant while 8% severe interactions were found which should be changed. Also 22 contra-indicated prescriptions were found with 10% occurrence (Figure 5).
| S. no | Name of antimicrobial | Amount prescribed | % Age of prescribing | Relevant info | % of N/A | |
|---|---|---|---|---|---|---|
| Availabe | N/A | |||||
| 1 | Cephradine | 9 | 2.49% | 7 | 2 | 22.22% |
| 2 | Cefaclor | 2 | 0.55% | 2 | 0 | 0% |
| 3 | Cefixime | 6 | 1.66% | 5 | 1 | 16.67% |
| 4 | Ceftriazone | 12 | 3.31% | 12 | 0 | 0% |
| 5 | Azithromycin | 30 | 8.29% | 24 | 6 | 20% |
| 6 | Clarithromycin | 14 | 3.87% | 9 | 5 | 35.71% |
| 7 | Amoxicilline | 6 | 1.66% | 6 | 0 | 0% |
| 8 | Vancomycin | 18 | 4.97% | 15 | 3 | 16.67% |
| 9 | Metronidazole | 83 | 22.93% | 57 | 26 | 31.32% |
| 10 | Ciprofloxacin | 52 | 14.36% | 34 | 18 | 34.62% |
| 11 | Levofloxacin | 37 | 10.22% | 26 | 11 | 29.73% |
| 12 | Moxifloxacin | 12 | 3.31% | 12 | 0 | 0% |
| 13 | Ampiclox | 11 | 3.04% | 9 | 2 | 18.18% |
| 14 | Amoxi-clave | 14 | 3.87% | 11 | 3 | 21.43% |
| 15 | Arthemether/Lumifantrine | 56 | 15.47% | 48 | 8 | 14.29% |
| 16 | Total | 362 | 100% | 277 | 85 | 23.48% |
Table 5: Antimicrobials prescribed.

Figure 5: Graph representing % of antimicrobials prescribed.
Inappropriate and indiscriminate use of antimicrobials and their combinations is a global problem causing a substantial economic burden on health care systems. Over prescribing is associated with increased side effects, excessive cost of the therapy, moreover it leads to emergence of resistant organisms, whereas under prescribing gives rise to treatment failure. Antimicrobial drug resistance refers to non-responsiveness of micro-organisms to an antimicrobial agent. One important reason for antimicrobial drug resistance is irrational use of FDCs. The present study was undertaken to evaluate rational use of antimicrobial FDCs in OPD of a tertiary care hospital.
There was lake of information about the corresponding patients in Govt; Hospitals’ prescriptions and many other Private clinics. This is the 1st step toward irrational prescribing, dispensing and use of drugs. Irrational prescribing is a global problem. Bad prescribing habits lead to ineffective and unsafe treatment, exacerbation or prolongation of illness, distress and harm to the patient, and higher costs. One of the major cause of the irrational prescription is poly-pharmacy. We found that 11% Rx were with only 2 drugs/Rx, 38.5% prescriptions were with 3 drugs/Rx, 29.5% with 4 drugs/Rx, 16% with 5 drugs/Rx and 5% prescriptions were with more than 5 drugs per encounter. While the average number of drugs per prescription is 3.67. Which is much higher than the proposed as this average is 1.3-2.2 in developed countries [15]. This will leads to Poly- Pharmacy.
We found that 21% prescriptions have multiple antimicrobial agents, which shows high degree of misuse of Antibiotics and this also leads to the microbial Resistance toward the Antibiotics. FDCs were also prescribed like Ampiclox, Augmentin and Artem with 71 prescriptions have only 1 FDCs/Rx, while 5 prescriptions with 2 FDCs/Rx. Overall FDCs prescription rate 38%. This is much higher rate which leads to the in-efficacy and also to microbial resistance.
Antibiotics should be use with high adherence to the prescriber information, as it cause serious consequences or it may not be effective. Like tetracyclines should not be given to the patient with milk because it causes decrease absorption and in turn ineffectiveness. For rational use of drug, it is necessary to prescribe rationally, which has minimum rate of drug-drug interactions and should be prescribed with proper interactions. But unfortunately in our cities (Lakki Marwat, D.I.K and Bannu) there is lake of such prescribing activity. These should be minimized to enhance the health of community.
Physicians must have a clear understanding of rational therapeutic use of antibiotics. They must be aware of the prevalence of various pathogens and resistance patterns in their hospital and exercise good judgment in selection of the antibiotic regimens. Irrationality can be addressed by use of guidelines, educational activities and surveillance at all level of health care. So, measures should be taken to avoid the inappropriate use of antibiotics. Drug utilization review programme must be carried out to study the rational use of antimicrobials.
The present study reveals that the pattern of prescriptions in terms of rationality of antimicrobials and FDCs remains poor. There is an urgent need to develop standards of antimicrobial drug prescriptions to avoid drug resistance. Educational interventions to promote rational use of antimicrobial agents and awareness of deleterious impact of irrational prescribing habit on the community and all members of the health care system are needed.
The situation of rational drug use indicators in the hospitals where the study was conducted is alarming. There is an urgent need for intervention to improve the situation. This study was conducted mainly at government hospitals and private clinics in Lakki Marwat, D.I. Khan and Bannu. It is recommended that this study should be conducted at a large scale level in order to have a clearer picture of the situation.
First and foremost, we humbly present our thanks to the Almighty ALLAH, the most merciful & beneficent for endowing us health, patience, knowledge, thought and opportunity to perceive higher ideals of life. We offer our humble gratitude to HOLY PROPHET (PEACE BE UPON HIM) who is forever a torch of guidance & knowledge for whole mankind.
We are thankful to our supervisor Dr Sir. Sheikh Abdur Rashid, (Asst Prof) faculty of pharmacy, Gomal university, D.I. Khan for their hard work, cooperative attitude and their precious time.
We express our feelings of admiration for our affectionate parents, brothers for their prayers, encouragement and support.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Khan FU (2024) To Evaluate the Prescribing Behaviour of Anti-Microbial in Lakki Marwat, Bannu and D.I. Khan Cities. J Pharm Care Health Syst. 11:336.
Received: 28-Apr-2020, Manuscript No. JPCHS-24-4027; Editor assigned: 01-May-2020, Pre QC No. JPCHS-24-4027 (PQ); Reviewed: 15-May-2020, QC No. JPCHS-24-4027; Revised: 15-May-2024, Manuscript No. JPCHS-24-4027 (R); Published: 12-Jun-2024 , DOI: 10.35248/2376-0419.24.11.336
Copyright: © 2024 Khan FU. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.