
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2024)Volume 14, Issue 1
Pesticides affect non-target organisms, the ecosystem including the atmosphere, soil, groundwater, surface water and the food web. This study was undertaken to investigate mancozeb toxicity on the Clarias gariepinus juveniles’ kidney, liver and spleen histoarchitecture. A total of 120 C. gariepinus with standard length and weight that ranged from 9.8 to 17.5 cm and 11 to 55 g were used for the experiment. Catfish juveniles were exposed to four sub-lethal concentrations (treatments) viz: 0.00, 20.55, 41.09 and 82.18 mg/L (Groups A-D) mancozeb fungicide. Each group was replicated three times and had 10 fish per replica summing up to 30 fish. The kidney of C. gariepinus exposed to lower concentrations showed vacuolar degeneration and necrosis of tubule lining cells, while the control group showed normal piscine kidney histoarchitecture. The liver of C. gariepinus exposed to lower concentrations showed increased lipid-like vacuolation, while the lower concentration during recovery period showed multifocal areas of hepatocellular necrosis with moderate infiltration of inflammatory leucocytes. The spleen of the control group and treatment group shows normal piscine splenic histoarchitecture throughout the duration period. The present investigation reveal that mancozeb was moderately to highly toxic to C. gariepinus and should be used with caution.
Mancozeb; Clarias gariepinus; Histopathology; Kidney; Liver; Spleen
In recent years, the high rate of increase in human population and rapid pace of industrialization have increase pesticide use and created problems on its disposal in waste waters [1-4]. These pesticides affect non-target organisms, the ecosystem including the atmosphere, soil, groundwater, and surface water and also food web [5-8].
Furthermore, most pesticides are rated to be extremely toxic when released to the environment; they accumulate in the living organism tissues and cause death [9]. Fish can accumulate toxic chemicals in their habitats using numerous parts [10]. Fungicide deposits end up in aquatic ecosystem and can be a fundamental link in evaluating environmental impact assessment for aquatic flora and fauna. The pesticides with higher statistical usage are insecticides, herbicides and fungicides [11-13].
Mancozeb is a synthetic ethylene (bis)-dithiocarbamates belonging to a subclass carbamate Fungicide [14,15]. Mancozeb is extensively utilized in agriculture to safeguard crops from a wide variety of fungal diseases [16,17]. Mancozeb applicators are prone to developing thyroid diseases [18,19]. Exposure routes and pathways to mancozeb are similar to ethylene (bis)- dithiocarbamates, maneb. Inhalation exposure of mancozeb can cause upper respiratory tract irritation; while infestation of mancozeb can result to nausea, headache, dizziness and diarrhea and chronic toxicity exposure can cause convulsions and coma [14]. Professional exposure to mancozeb occurs accidentally to agricultural workers, vineyard workers, or horticulturist [20]. Anthropological mismanagement throughout different stages of pesticide manufacture, shipping, packaging and usage also harms other species [21]. Environmental stressors can induce morphological indices, haematological parameters, biochemical parameters, physiological and antioxidant responses in fish [22,23]. Several tissues and organs of fishes are continually used as biomarker of fish contamination due to their specific features. All these tissues have imperative purposes in life of a fish and their liver has metabolic pathways for eradicating the harmful compounds [24]. Pesticides breakdown slowly and may be more accessible to aquatic animals [25]. Mancozeb like other carbamate can damage the nervous system. It inhibits the function of neurotransmitter Acetylcholinesterase (AchE) in the central nervous system of insects by its primary metabolites ethylenethiourea and carbon disulfide. AchE catalyses the hydrolysis of the acetylcholine to acetic acid and choline to slowdown the nerve stimulation. As a result, acetylcholine concentration remains high in the junction that gives rise to regular stimulation to the muscle resulting to fatigue and tetany followed by countless categories of poisoning symptoms, respiratory failure and death [26]. Generally, histopathological analysis appears to be very sensitive parameter and is vital to evaluate cellular changes that may occur in target organs such as kidney, liver and spleen [27-29]. This study was undertaken to investigate mancozeb toxicity on the Clarias gariepinus juveniles’ kidney, liver and spleen histoarchitecture.
Procurement of experimental fish
A total of 120 Clarias gariepinus juveniles with standard length and weight that ranged from 9.8 cm to 17.5 cm and 11 g to 55 g were procured from Freedom Fisheries Limited, University Market Road, Nsukka, Enugu State, Nigeria and was transported to Fisheries Wet Laboratory, Department of Zoology and Environmental Biology, University of Nigeria, Nsukka. The catfishes were disinfected with 0.05% potassium permanganate (KMnO4) for 2 minutes to avoid any dermal infections and were allowed to acclimatize to laboratory conditions for 2 weeks in plastic tanks of 300 Liter (L) capacity. Catfishes were fed daily with food (Aqua-feed commercial feed size 3 mm) contaminating 40% crude protein twice daily at 2%-3% body weight. Food, faecal matter and other wastes were siphoned off and water was changed weekly to reduce ammonia content in water. Dead fishes were removed with forceps to avoid possible deterioration of the water quality. During acclimatization, the water was changed weekly with well aerated tap water.
Sources of the test compound
The commercial formulation of Mancozeb 80% WP (Z- FORCE®) weighing 50 g with batch number 01062018 was purchased from manufacturer (Jubaili Agrotec Limited Abuja, Nigeria) and stored at room temperature.
Experimental design for sub lethal exposure
The experimental design for sub-lethal exposure consists of 120 fish for four groups of 0.0, 20.55, 41.09 and 82.18 mg/L (A-D), each with three replicates. Each tank contained 10 litre dechlorinated tape water served as the control, while the three other treatments were exposed to water containing 20.55, 41.09 and 82.18 mg/L of mancozeb corresponding to 1/20, 1/10 and 1/5 of the 96 hours LC50 value (410.90 mg/L) that was derived from acute toxicity experiment. The experimental duration lasted 28 days during which the fish was fed with small quantity of feed approximately 1% of their total body weight about an hour before the test solution were renewed daily. The feeding was to avoid mortality and cannibalism.
On each sampling day (1, 7, 14, 21 and 28) three fishes from each of the treatment groups were sacrificed after anesthesizing with Tricaine methanesulfonate (MS-222) to minimize stress. The organs (Kidney, liver, gill and spleen) were harvested from viscera and quickly rinsed in cold 0.9% sodium chloride solution. At, the end of the sub-lethal exposure, the remaining fish in each of the concentrations were withdrawn from the exposure of chemical and were placed in chemical free water in which further observation were made after 7 days of the withdrawal.
Histopathology of the kidney, liver and spleen
One fish was sampled from each replicate, three fishes per treatment at days 1, 7, 14, 21 and 28. The fishes were sacrificed and dissected under anesthesia (MS-222), and the organs (kidney, liver and spleen) were harvester from the viscera.
Tissue preparation
Organs were fixed in 10% phosphate buffered formalin for 48 hours. The organs were trimmed, dehydrated in 4 grades of alcohol ranges (70%, 80%, 90% and absolute alcohol) and cleared in 3 grade ranges of xylene. The organs were embedded in molten wax (60°C). The molten organs were mounted on sectioning blocks and cut into 5 µm thick sections with a microtome. The sections 5 µm thick were floated in water bath at 60°C for 30 minutes and mounted albuminized glass slides. 5 µm thick tissue sections were cleared in 3 grades of xylene and rehydrated in 3 grades of alcohol ranges (90%, 80%, 70%) respectively. The tissues were subsequently stained with haematoxylin for 15 minutes and blued with ammonium chloride. Differentiation were performed with 1% acid alcohol and counterstained with eosin. Permanent were prepared using DPX and cover slip.
Slide examination
The prepared slides were observed on motic camera attached to binocular light microscope. The slides will be viewed with different objective lenses (X 4, X 10, and X 40) and photomicrographs were taken for more analysis.
Histopathology of the kidney of Clarias gariepinus exposed to different concentration of mancozeb on day 1
The photomicrographs of kidney cells exposed to different concentration of mancozeb and the control on day 1 showed normal piscine nephron histoarchitecture (Figure 1).
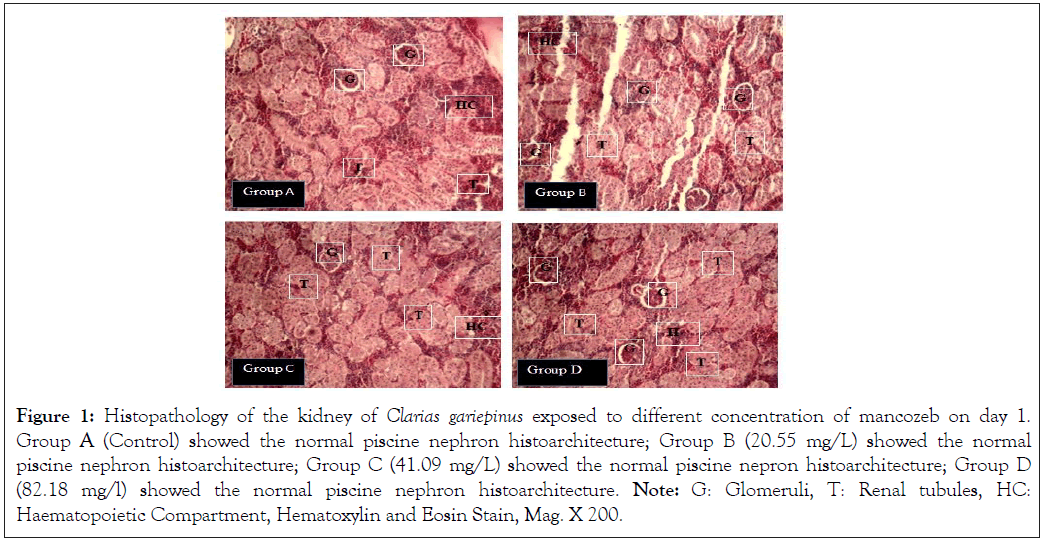
Figure 1: Histopathology of the kidney of Clarias gariepinus exposed to different concentration of mancozeb on day 1. Group A (Control) showed the normal piscine nephron histoarchitecture; Group B (20.55 mg/L) showed the normal piscine nephron histoarchitecture; Group C (41.09 mg/L) showed the normal piscine nepron histoarchitecture; Group D (82.18 mg/l) showed the normal piscine nephron histoarchitecture. Note: G: Glomeruli, T: Renal tubules, HC: Haematopoietic Compartment, Hematoxylin and Eosin Stain, Mag. X 200.
Histopathology of the kidney of Clarias gariepinus exposed to different concentration of mancozeb on day 7
The photomicrographs of kidney cells exposed to different concentration of mancozeb and the control (Groups A, C and D) on day 7 showed normal piscine nephron histoarchitecture (Figure 2). Whereas group kidney cells from group B showed necrosis degeneration of renal tubules, inflammation of the haematopoitic tissue, shrinkage of tube lumen, haemorrhage and contraction of the glomeruli (black arrows).
Figure 2: Histopathology of the kidney of Clarias gariepinus exposed to different concentration on day 7. Group A (Control) showed the normal piscine nephron histoarchitecture; Group B (20.55 mg/L) showed vacuolar degeneration and necrosis of the renal tubule lining cells; Group C (41.09 mg/L) showed the normal piscine nephron histoarchitecture; Group D (82.18 mg/L) showed the normal piscine nephron histoarchitecture. Note: G: Glomeruli, T: Renal tubules, HC: Haematopoietic Compartment, Black arrows: Renal tubules lining cells, Hematoxylin and Eosin Stain, Mag. X 200.
Histopathology of the kidney of Clarias gariepinus exposed to different concentration of mancozeb on day 14
The photomicrographs of kidney cells exposed to different concentration of mancozeb, and the control (Groups A, C and D) on day 14 showed normal piscine nepron histoarchitecture (Figure 3). Whereas, kidney cells from group B had necrosis degeneration of renal tubules, inflammation of the haematopoitic tissue, shrinkage of tube lumen, haemorrhage and contraction of the glomeruli (black arrows).
Figure 3: Histopathology of the kidney of Clarias gariepinus exposed to different concentration of mancozeb on day 14. Group A (Control) showed the normal piscine nephron histoarchitecture; Group B (20.55 mg/L) showed vacuolar degeneration and necrosis of the renal tubule lining cells; Group C (41.09 mg/L) showed the normal piscine nephron histoarchitecture; Group D (82.18 mg/l) showed the normal piscine nephron histoarchitecture. Note: G: Glomeruli, T: Renal tubules, HC: Haematopoietic Compartment, Black arrows: Renal tubules lining cells. Hematoxylin and Eosin Stain, Mag. X 200.
Histopathology of the kidney of Clarias gariepinus exposed to different concentration of mancozeb on day 21
The photomicrographs of kidney cells exposed to different concentration of mancozeb, and the control (Groups A, C and D) on day 21 had normal piscine nephron histoarchitecture (Figure 4). Whereas, kidney cells from group B had necrosis degeneration of renal tubules, inflammation of the haematopoitic tissue, shrinkage of tube lumen, haemorrhage and contraction of the glomeruli (black arrows).
Figure 4: Histopathology of the kidney of Clarias gariepinus exposed to different concentration on day 21. Group A (Control) showed the normal piscine nephron histoarchitecture; Group B (20.55 mg/L) showed vacuolar degeneration and necrosis of the renal tubule lining cells; Group C (41.09 mg/L) showed the normal piscine nephron histoarchitecture; Group D (82.18 mg/l) showed the normal piscine nephron histoarchitecture. Note: G: Glomeruli, T: Renal tubules, HC: Haematopoietic Compartment, Black arrows: Renal tubules lining cells, Hematoxylin and Eosin Stain, Mag. X 200.
Histopathology of the kidney of Clarias gariepinus exposed to different concentration of mancozeb on day 28
The photomicrographs of kidney cells exposed to different concentration of mancozeb, and the control (Groups A and D) on day 28 had normal piscine nephron histoarchitecture (Figure 5). Whereas, kidney cells from group B and C revealed mild vacuolar degeneration and necrosis of the renal tubule lining cells, inflammation of the haematopoitic tissue, shrinkage of tube lumen, haemorrhage and contraction of the glomeruli (black arrows).
Figure 5: Histopathology of the kidney of Clarias gariepinus exposed to different concentration on day 28. Group A (Control) showed the normal piscine nepron histoarchitecture; Group B (20.55 mg/L) showed mild vacuolar degeneration and necrosis of the renal tubule lining cells; Group C (41.09 mg/L) showed mild vacuolar degeneration and necrosis of the renal tubule lining cells; Group D (82.18 mg/l) showed the normal piscine nepron histoarchitecture. Note: G: Glomeruli, T: Renal tubules, HC: Haematopoietic Compartment, Black arrows: Renal tubules lining cells, Hematoxylin and Eosin Stain, Mag. X 200.
Histopathology of the liver of Clarias gariepinus exposed to different concentration of mancozeb on day 1
The photomicrographs of liver cells exposed to different concentration of mancozeb and the control on day 1 had hepatocytes, parenchyma and sinusoids indicating normal piscine liver cells histoarchitecture (Figure 6).
Figure 6: Histopathology of the liver of Clarias gariepinus exposed to different concentration on day 1. Group A (Control) showed the normal histoarchitecture of the piscine liver cells; Group B (20.55 mg/L) showed the normal histoarchitecture of the piscine liver cells; Group C (41.09 mg/L) showed the normal histoarchitecture of the piscine liver cells; Group D (82.18 mg/L) showed the normal histoarchitecture of the piscine liver cells. Note: V: Central vein, P: Portal area, Mag. X 200.
Histopathology of the liver of Clarias gariepinus exposed to different concentration of mancozeb on day 7
The photomicrographs of liver cells exposed to different concentration of mancozeb, and the control on day 7 had hepatocytes, parenchyma and sinusoids indicating normal piscine liver cells histoarchitecture (Figure 7).
Figure 7: Histopathology of the liver of Clarias gariepinus exposed to different concentration on day 7. Group A (Control) showed the normal histoarchitecture of the piscine liver cells; Group B (20.55 mg/L) showed the normal histoarchitecture of the piscine liver cells; Group C (41.09 mg/L) showed the normal histoarchitecture of the piscine liver cells; Group D (82.18 mg/L) showed the normal histoarchitecture of the piscine liver cells. Note: V: Central vein, P: Portal area, Mag. X 200.
Histopathology of the liver of Clarias gariepinus exposed to different concentration of mancozeb on day 14
The photomicrographs of the kidney cells exposed to different concentration of mancozeb, and the control on day 14 (Groups A, C and D) and showed normal histoarchitecture of the piscine liver cells. Whereas, liver cells from group B had increased lipid- like vacoulation (black arrows) (Figure 8).
Figure 8: Histopathology of the liver of Clarias gariepinus exposed to different concentration on day 14. Group A (Control) showed the normal histoarchitecture of the piscine liver cells; Group B (20.55 mg/L) showed increased lipid-like vacuolation; Group C (41.09 mg/L) showed the normal histoarchitecture of the piscine liver cells; Group D (82.18 mg/L) showed the normal histoarchitecture of the piscine liver cells. Note: V: Central vein, P: Portal area, Black arrow: Lipid-like vacoulation, Mag. X 200.
Histopathology of the liver of Clarias gariepinus exposed to different concentration of mancozeb on day 21
The photomicrographs of kidney cells exposed to different concentration of mancozeb, and the control (Groups A, B and D) on day 21 had normal histoarchitecture of the piscine liver cells (Figure 9). Whereas, liver cells from group C had increased lipid like vacoulation (black arrows).
Figure 9: Histopathology of the liver of Clarias gariepinus exposed to different concentration on day 21. Group A (Control) showed the normal histoarchitecture of the piscine liver cells; Group B (20.55 mg/L) showed the normal histoarchitecture of the piscine liver cells; Group C (41.09 mg/L) showed increased lipid-like vacuolation; Group D (82.18 mg/L) showed the normal histoarchitecture of the piscine liver cells. Note: V: Central vein, P: Portal area, Black arrow: Lipid like vacoulation, Mag. X 200.
Histopathology of the liver of Clarias gariepinus exposed to different concentration of mancozeb on day 28
The photomicrographs of kidney cells exposed to different concentration of mancozeb, and the control (Groups A, C and D) on day 28 had normal histoarchitecture of the piscine liver cells (Figure 10). Whereas, liver cells group B revealed multifocal areas of hepatocellular necrosis with leucocytes infiltration (black arrows).
Figure 10: Histopathology of the liver of Clarias gariepinus exposed to different concentration on day 28. Group A (Control) showed the normal histoarchitecture of the piscine liver cells; Group B (20.55 mg/L) showed multifocal areas of hepatocellular necrosis with moderate infiltration of inflammatory leukocytes; Group C (41.09 mg/L) showed the normal histoarchitecture of the piscine liver cells; Group D (82.18 mg/L) showed the normal histo-architecture of the piscine liver cells. Note: V: Central vein, P: Portal area, Black arrow: Lipid like vacoulation, Mag. X 200.
Histopathology of the spleen of Clarias gariepinus exposed to different concentration mancozeb on day 1
The photomicrographs of spleen cells exposed to different concentration of mancozeb, and the control on day 1 had piscine histoarchitecture of the spleen (Figure 11). The white pulp, rep pulp, ellipsoid and haemosiderim pigment were not degenerated or necrotized.
Figure 11: Histopathology of the spleen of Clarias garipinus exposed to different concentration of mancozeb on day 1. Group A (Control) showed the normal piscine splenic histoarchitecture; Group B (20.55 mg/L) showed the normal piscine splenic histoarchitecture; Group C (41.09 mg/L) showed the normal piscine splenic histoarchitecture; Group D (82.18 mg/) showed the normal piscine splenic histoarchitecture. Note: WP: White Pulp, RP: Red Pulp, E: Ellipsoid, H: Haemosiderin pigment, Mag. X 200.
Histopathology of the spleen of Clarias gariepinus exposed to different concentration mancozeb on day 7
The photomicrographs of spleen collected on day 7 (Plate 12) exposed to different concentration of Mancozeb on day 7 and the control revealed piscine histoarchitecture of the spleen. There was no disintegration of the white pulp, red pulp, ellipsoid, and haemosiderim pigment (Figure 12).
Figure 12: Histopathology of the spleen of Clarias gariepinus exposed to different concentration on day 7. Group A showed the normal piscine splenic histoarchitecture; Group B (20.55 mg/L) showed the normal piscine splenic histoarchitecture; Group C (41.09 mg/L) showed the normal piscine splenic histoarchitecture; Group D (82.18 mg/L) showed the normal piscine splenic histoarchitecture. Note: WP: White Pulp, RP: Red Pulp, E: Ellipsoid, H: Haemosiderin pigment, Mag. X 200.
Histopathology of the spleen of Clarias gariepinus exposed to different concentration mancozeb on day 14
The photomicrographs of spleen cells on day 14 exposed to different concentration of mancozeb on day 14 and the control indicated that the piscine histoarchitecture of the spleen was not distorted (Figure 13). There was no necrosis and disintegration of the white pulp, red pulp, ellipsoid, and haemosiderin pigment.
Figure 13: Histopathology of the spleen of Clarias gariepinus exposed to different concentration of mancozeb on day 14. Group A (Control) showed the normal piscine splenic histoarchitecture; Group B (20.55 mg/L) showed the normal piscine splenic histoarchitecture; Group C (41.09 mg/L) showed the normal piscine splenic histoarchitecture; Group D (82.18 mg/ L) showed the normal piscine splenic histoarchitecture. Note: WP: White Pulp, RP: Red Pulp, E: Ellipsoid, H: Haemosiderin pigment, Mag. X 200.
Histopathology of the spleen of Clarias gariepinus exposed to different concentration of mancozeb on day 21
The photomicrographs of spleen cells exposed to different concentration of mancozeb and the control on day 21 had normal piscine spleen histoarchitecture (Figure 14). Normal white pulp, red pulp, ellipsoid, and haemosiderin pigment were recorded.
Figure 14: Histopathology of the spleen of Clarias gariepinus exposed to different concentration of mancozeb on day 21. Group A (Control) showed the normal piscine splenic histoarchitecture; Group B (20.55 mg/L) showed the normal piscine splenic histoarchitecture; Group C (41.09 mg/L) showed the normal piscine splenic histoarchitecture; Group D (82.18 mg/L) showed the normal piscine splenic histoarchitecture. Note: WP: White Pulp, RP: Red Pulp, E: Ellipsoid, H: Haemosiderin pigment, Mag. X 200.
Histopathology of the spleen of Clarias gariepinus exposed to different concentration of mancozeb on day 28
The photomicrographs of spleen cells exposed to different concentration of mancozeb on day 28 and the control revealed piscine histoarchitecture of the spleen (Figure 15).
Figure 15: Histopathology of the spleen of Clarias gariepinus exposed to different concentration of mancozeb on day 28. Group A (Control) showed the normal piscine splenic histoarchitecture; Group B (20.55 mg/L) showed the normal piscine splenic histoarchitectur; Group C (41.09 mg/L) showed the normal piscine splenic histoarchitecture; Group D (82.18 mg/L) showed the normal piscine splenic histoarchitecture. Note: WP: White Pulp, RP: Red Pulp, E: Ellipsoid, H: Haemosiderin pigment, Mag. X 200.
The present study revealed that the histopathology of the kidney of Clarias gariepinus collected on day 1 exposed to different concentration of mancozeb showed normal piscine nephron histoarchitecture. The kidney controls have intact nephrons and interstitial tissues. The normal piscine architecture of the kidney exposed to mancozeb was both time and concentration dependent. In the present study histopathological changes revealed necrosis degeneration of renal tubules, inflammation of the haematopoietic tissue, shrinkage of tube lumen and haemorrhage of kidney cells exposed to sub lethal concentration of mancozeb on day 7 and 21 (group B), day 14 (group A and B), day 28 (group B and C). This suggests that fish kidney suffered damage after exposure to mancozeb. Lesions recorded in the kidney indicate nephrotoxicity caused by the tested compound and its metabolites since kidneys major role is to eliminate pesticides. This agreed with the report of Choudhury, et al. [30], on the exposure of Channa puntatus to mancozeb that revealed the formation of vacuoles of interstitial dilation, shrinkage of the bownman’s capsule, hypertrophy of distal convoluted tubules and swelling of renal tubules on day 30. Onchohynchus mykiss exposed to carbosulfan, benomyl and propineb revealed tubular cell necrosis, renal degeneration, necrotic haemopoitic tissue and contraction of the glomeruli [31]. Similarly, Altinok, et al. [32], reported the effects of methiocarb on rainbow trout kidney to incude tubular necrosis and renal tubules filled with eosinophilic materials. Ortiz, et al. [33], reported vacuolisation of tubular epithelial cells, necrosis, and desquamation of piscine kidney exposed to lindane. Cyprinus carpio exposed to deltametrin showed degeneration of epithelial cells of the renal tubule and poicnoic nuclei in the haematopoitic tissue. Catla catla exposed to bisphenol-A revealed obliteration of bowman’s space, shrinkage, degeneration of tubules and glomerulus [34]. Oreochromis niloticus exposed to bisphenol-A showed degeneration of glomerulus and enlarged Bowman’s capsule [35]. Vinodhini, et al. [36], reported Cyprinus carpio exposed to heavy metal showed macrophages with lipofuscin granules accumulated in the affected cells. Anabas testudineus exposed to phosphamidon revealed shrinkage of renal tubules and glomeruli, presence of melano-macrophage centres and degeneration of renal tubules [37].
Histopathology of fish organ exposed to mancozeb in this study had more effect on the kidney than on the liver and spleen. Kidney is referred to as a crucial organ of excretion and it may be prone to pathological effects induced by pesticides. This is because the kidney detoxifies and removes wastes from the body [38]. It is not only mancozeb fungicide; any pesticide can impair the piscine histoarchitecture. The shrinkage of renal tubules may be due to the reaction of pesticides on the wall of renal tubules or due to the osmotic imbalance which slowly decreases the excretory surface area. Later, it causes the decline of excretion and gradually leads to fish mortality. Patel, et al. [39], reported on Oreochromis mossambicus and Labeo rohita exposed to imidacloprid and curzate had kidney with severe necrosis of tubular epithelial cells thickening of the Bowman’s capsule and shrinkage of the glomeruli along with severe degenerative necrotic changes in the renal tubules with focal areas of necrosis and haemorrhage. Tubular degeneration and dilation of capillaries was the most common pathological effects observed on numerous fish species histoarchitecture induced by pesticides and other hazardous chemicals [40]. Similarly, Odo et al. [41], reported on Clarias gariepinus exposed to potassium permanganate revealed distortion of histoarchitecture and histopathological changes. This was in agreement and disagreement with Odo et al. [42], reported on Clarias gariepinus exposed to cyperdicot and vitamin E in kidney histopathology, in which cyperdicot revealed vacoular degeneration of glomerular, increased Bowman’s capsule space, dilation of tubules lumens, shrinkage of glomeruli. This was similar to Mataqueiro, et al. [43], reported that Piaractus mesopotamicus exposed to trichlorfon an organophosphate insecticide.
In this study the liver of the control treatment had hepatocytes, parenchyma and sinusoids. The liver cells collected on day 1 and 7 showed normal liver histoarchitecture [44]. Also in this study the histopathology of the liver showed on effect in group B on day 14, group C on day 21 had increased lipid-like vacoulation [34]. Group B day 28 showed multifocal areas of hepatocellular necrosis with leucocytes infiltration [45]. This may be due to adverse reaction of the mancozeb on the hepatocytes during the study period. The histoarchitecture of Clarias gariepinus liver exposed to mancozeb was duration and concentration dependent. Most of the hepatocytes appear to contain large amounts of lipid like substances within the cytoplasm. The qualitative liver histology revealed a high hepatocyte size in Clarias gariepinus exposed to mancozeb as a result of high lipid content [46]. This is not uncommon in captive fishes and is due to the fact that in the captive state energy intake exceeds the demand of metabolism resulting to energy storage in the liver as glycogen or lipids [47]. The liver of Clarias gariepinus exposed to mancozeb in the present study had slight decline. Toxic chemicals affect the liver organ; it plays a major function in breaking down detrimental compounds in the body [48,49]. This finding agreed with the report that exposure of C. puntatus to mancozeb caused degeneration of the hepatic tissues and formation of vacuoles as seen from day 15 to day 30 post- treatment. Other authors reported high frequency of hepatic hypertrophy, hepatocellular carcinoma, hepatic tumors, hepatic cancer and debilitating liver diseases in various animals [50-53]. It had earlier reported similar result when Clarias gariepinus was exposed to 96 hours acute toxicity of paraquat dichloride. Similarly, Makinde, et al. [53], reported hepatocyte necrosis in Clarias gariepinus exposed to 2,4-D amine. Mostakim, et al. [54], reported similar alterations in the histoarchitecture of the liver and kidney of silver barb after chronic exposure to quinophos. In this study, fish exposed to mancozeb day 28 (group B) showed multifocal areas of hepatocellular necrosis and moderate infilteration of inflammatory leucocytes. This finding were in line with the finding of Mela, et al. [55], Rhamdia quelen liver histoarchitecture exposed to atrazine revealed leukocyte, infiltration, hepatocyte vacoulation like steatosis and necrosis areas leading to lesion index levels in all tested concentration. Similarly, this finding was in agreement or less with recorded fish liver exposed to paraquat and malathion [56]. Figueire- Fernandez, et al. [57], reported about Oreochromis niloticus exposed to cupper for 21days. In contrast, Makinde et al. [54], reported that Clarias gariepinus juveniles exposed to acute concentration of 2, 4 D-amine beyond 96 hours may suffer severe pathological death. Liver necrosis of fishes can be linked to the damage of the hepatic blood vessels which may result in the complete dissolution of the hepatocytes [58]. Histological biomarkers are adopted to access the health conditions of fishes and to indicate an environmental hazard. This was in agreement with Odo, et al. [41], report on Clarias gariepinus exposed to potassium permanganate that had histoarchitectural distortion and histopathologoical changes in the liver.
Lastly, Odo, et al. [42], reported on effect of cyperdicot and vitamin E on liver was in agreement and disagreement indicating that cyperdicot effect on liver revealed slight vacuolated cells showing evidence of degenerated fatty degeneration and also similar to Gambus affinis, Corydoras paleatus, P. gonionotus exposed to paraquate and dimethoate as reported by Fanta, et al. [59].
In this study, the histopathology of the spleen of C. gariepinus exposed to different concentration of mancozeb from day 1 to 28 (7 days recovery) revealed no histological changes in piscine histoarchitecture of the spleen when compared to control. There was no lesion observed in the spleen and no concentration related effect observed on piscine histoarchitecture exposed to mancozeb. In the present study, the concentrations of mancozeb did not cause any histopatholological effect. Onchohynchus mykiss exposed to methiocarb revealed no lesions in spleen, kidney and liver histoarchitecture for 21 days [44].
Some authors have documented the effects of pesticides on piscine spleen histoarchitecture Hypophthalmicthys molitrix, Oreochromis niloticus exposed to deltametrin revealed necrosis and significant changes in number of melanomacrophage centres compared to control [60,61]. As a component of fish immune system, the spleen is a major pheripheral lymphoid organ which performs antigen trapping function regulate the immune response and resist foreign antigens [62,63].
C. gariepinus may be resistant to mancozeb due to possession of gene that allows it to break the chemical down into harmless substances. This may be why mancozeb had no effect on the catfish spleen histoarchitecture. Histological analysis is a vital bio-indicator that can be used to determine cellular changes in organs such as kidney, liver and spleen [64-67].
The present study revealed that C. gariepinus exposed to various sub-lethal concentrations of carbamate fungicide, mancozeb elicited harmful effects on the kidney, liver except spleen histoarchitecture. Further studies pertaining to the effect of mancozeb exposure on spleen as a biomarker will boost our understanding of other ichthyofaunal species histoarchitecture. Monitoring the utilization of mancozeb in controlling or eradicating fungal diseases for vegetables, seeds and fruits therefore should be advocated worldwide. Dithiocarbamates e.g., Mancozeb is regarded as a fungicide of low toxicity.
Authors appreciate Prof. Gregory Ejikeme Odo of Department of Zoology and Environmental Biology, University of Nigeria, Nsukka, Nigeria, and my Parents Chief and Lolo Nwosu for their mentorship and contributions during this study.
The authors declare no conflicts of interest.
This research was funded by Mr. Francis Chijioke. There was no external sponsorship.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar][
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Chijioke F, UU O, Odinakachukwu NL, Chinedu NO, Benjamin AC, JC I, et al. (2024) Toxicity of Mancozeb on the African Catfish Clarias gariepinus Juveniles Kidney, Liver and Spleen Histoarchitecture. J Clin Toxicol. 14:555.
Received: 18-Jan-2024, Manuscript No. JCT-24-29237; Editor assigned: 22-Jan-2024, Pre QC No. JCT-24-29237 (PQ); Reviewed: 05-Feb-2024 Revised: 12-Feb-2024, Manuscript No. JCT-24-29237 (R); Published: 19-Feb-2024 , DOI: 10.35248/2161-0495.24.14.555
Copyright: © 2024 Chijioke F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.