
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2023)Volume 13, Issue 3
Environmental impurities effectively served as bio-indicators of pollution have generally used to assess the quality of the aquatic zone. The present study investigated zebrafish fenvalerate exposure to a range (20 μg/L-100 μg/L) was made. In the 24th h of exposure at 20 μg/L, 55% mortality was observed. After 96th h, 82% death was obtained. In 40 μg/L, 62% mortality was registered after 24 h and further increased to 94% after 96 h of exposure. At 60 μg/L, 71% mortality was noted initially and it raised 99% at the 96th hour of the trial. In 80 μg/L the mortality rate ranged from 79% to 100%. Finally, in 72 h and 96 h, all fishes died and registered 100% mortality in 80 μg/L and 100 μg/L of fenvalerate concentration. The sub-lethal concentration induced biochemical changes which shown that normal fish gill protein was 120 ± 1.22 mg/g and it reduced to 118.31 ± 0.5 mg/g, 117.4 ± 1.8 mg/g, 114.3 ± 2.1 mg/g and 110 ± 1.4 mg/g, after 24 h, 48 h, 72 h and 96 h respectively. The total protein in muscle declined after 96 h treatment. In the control fish, total muscle protein was 80.2 ± 1.3 mg/g and it minimized to 78.4 ± 2.4 mg/g, 71.4 ± 2.2 mg/g in subsequent exposure of LC50. After 96 h, muscle protein was 60 mg/g ± 2.7 mg/g. In wet weight, control muscle lipid was 4 ± 0.46%. It has decreased (3.1 ± 0.12%) in the 96 h. In comparison, lipid of gill depleted from 6.5 ± 0.22% to 2.5 ± 0.31%. Polyacrylamide gel electrophoresis revealed variations in protein bands between the control and experimental group. Fenvalerate inhibited protein biosynthesis and induced stress by synthesis of stress proteins.
Biochemical changes; Exposure; Induced toxicity; Lethal rate; Mortality
Pesticides are the biological toxicants which are commonly required by the man to kill the pests, insects and also against various disease causing organisms. In recent years, the applications of pesticides became an indispensable and integral part of agriculture throughout the world. The severe application of these pesticides in agricultural operations has dangerously changed the ecological balance of various non target organisms like fishes. Environmental pollutants such as metals, pesticides and other organic supplies cause high menace to various aquatic organisms including fishes. They are the bio indicators of pollution in environment and can be generally used for the assessment of the quality of aquatic environment. Since fishes are directly open up the elements of various chemicals through surface runoff or indirectly by the food chain of ecosystem. The environmental aspects like pH level, atmospherics temperature and dissolved oxygen play a crucial task to enhance pesticide toxicity due to the presence of various residual molecules. The unhindered use of farming pesticides has fascinated ecologists to weigh up the impacts of noxious elements on natural communities [1].
The awareness of insect repellents has established more in aquatic flora and fauna rather than various earthly systems. The effect taken as a whole proved more in aquatic habitat regarding pesticides. More non-target organisms are affected by detrimental supplies are transported to greater distances in the hydrosphere. There are about 234 classes of pesticides under usage in India in which 24 are applied widely. Huge quantity of insecticides released into the earth by no means finds their target organisms and washed into aquatic environment. In past few decades, loads of farming and industrial noxious wastes form water sinks lead to global environmental issues [2].
Fenvalerate was first formulated for agricultural use in 1974, approved as a termiticide in 1987 and added as an alternative to cyclodiene termiticides. Principally in India, cotton and vegetables pests control were done by this chemical. The most sensitive pesticides impair immuno-competent organs especially liver and kidney of fishes. Assessment of the potential role of fenvalerate is essential to recognize its consequences on aquatic organisms; their immune suppressing ability and impacts on growth for finding suitable mitigation, amelioration or prophylactic measures [3].
Most of the commercial pesticides were reported to have high toxicity towards non-target aquatic organisms including fish and was extensively studied by Cao, et al. Hence synthetic pyrethroids were reported as one of the important contributors of aquatic pollution and extremely toxic to aquatic organisms. It could be noted that any impact of pesticides in fish subsequently affects humans through the food web because fish serves as an important food source of humans. Tarkhani, et al., determined the acute toxicity of diazinon and deltamethrin Danio rerio, where the LC50 values observed were indicated that mortality of zebrafish exposed to the pesticides severely increased with increasing concentration and time of treatment and further showed that zebrafish was highly sensitive to lower values of deltamethrin than diazinon. Sapanadevi and Gupta studied the noxious nature of lindane, deltamethrin and atrazine to the beginning stages of zebrafish (Brachydanio rerio) and observed that the fingerling stages were less sensitive to the pesticide, lindane, however, in the case of deltamethrin, atrazine the subjected larvae were more sensitive. Mohapatra, et al., reviewed fenvalerate induced improvement by supplementing dietary Probiotics in Labeo rohita in which total protein content was reduced notably in the fenvalerate exposed fish than that of fish supplemented with probiotics [4].
Fenvalerate is effective against various aquatic organisms and is also used in animal husbandry and public health. When this fenvalerate is washed down with running water it reaches to various water bodies and brings out toxic effect on various aquatic animals. To assess the significant role of pesticides such as fenvalerate, it is essential to find out their impact on aquatic organisms in growth and immune suppressing ability for finding suitable prophylactic and mitigation measures. Fenvalerate is absorbed at the elevated rate through the gills because of lipophilicity. However, fishes have a reduced capability to expel and metabolise fenvalerate and thus are highly susceptible even to very low quantity of the pesticide. These effects noted in the pyrethroid pesticide geared up to formulate the present study to measure the toxicity and its effects. Their biochemical changes and protein expression in muscle and gills due to pyrethroid insecticide fenvalerate toxicity to a fresh water zebra fish, Danio rerio were also carried out and evaluated [5].
Experimental animal collection
Freshwater zebrafish (Danio rerio) was gathered from an aquarium at Nagercoil, Kanyakumari district and Tamilnadu, India. Experimental animals were transported to the laboratory for further toxicity assays and ensured aeration during transportation (Figure 1).

Figure 1: External view of Danio rerio-entire.
Distinctiveness of experimental fishes
Zebrafish (Danio rerio) is a widespread and handy scientific model life form for studies of vertebrate improvement and gene function. Its importance has been forwarded by genetic screenings. The Zebrafish Information Network (ZFIN) has online database of genetic, genomic and developmental information dedicated for this genus. D. rerio is one of the few fish species sent into space. The zebrafish enclose numerous compensations for scientists for the reason that it is positioned as a model biological system. It has fully sequenced genome with identifiable, obvious developmental qualities. Its embryonic development is rapid and its embryos are relatively transparent, large, robust and able to develop outside their mother. They have parallel mammalian evolutionary traits and mutant strains characterized for human toxicity testing. They have a diurnal sleep cycle with similarities to mammalian sleep behavior. Some disadvantages to their scientific use are the absence of a standard diet and the presence of small differences between zebrafish and mammals in the roles of some genes related to human disorders [6].
Maintenance and culture of zebrafish
Freshwater zebrafish (D. rerio) was kept with 12 h/12 h light and dark cycle in a glass tank. The glass aquarium (60 cm × 30 cm × 25 cm) was maintained with dechlorinated water. Daily two times they were fed with freeze dried tubefex worms. About 20 animals were maintained for each set of experiment and acclimatized prior to the experiment at 28°C ± 2°C.
Fenvalerate and its structure
It is a broad spectrum pyrethroid with neurotoxic effects on insects. Fenvalerate is the approved common name by International Organization for Standardization (ISO) for (RS)-α-cyano-3-phenoxybenzyl (RS)-2-(4-chlorophenyl)-3-methylbutyrate. International union of pure and applied chemistry recognized this chemical and included under the chemical abstracts service number 51630-58-1. Fenvalerate is a racemic mixture of four stereoisomers ((2S, αS), (2S, αR), (2R, αS) and (2R, αR)) found two chiral centers in just about equal proportions (Figure 2) [7].
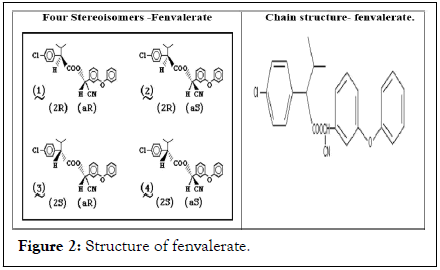
Figure 2: Structure of fenvalerate.
Effect of fenvalerate on zebrafish
In the laboratory condition, the fishes were acclimatized for 15 days and made into a control group and five investigational groups (Figure 2). Various concentrations of technical grade fenvalerate (Merck, Bangalore, India, 99.9% purity) were added to investigational groups (20 μg/L to 100 μg/L). To the control group, pesticide was not added. Experiment was carried out up to 96 h. Mortality observations were done after 24 h, 48 h, 72 h and 96 h correspondingly. Dead fish was counted, registered and removed from the tank immediately. Finally the percentage mortality was tabulated.
Biochemical impact of sub-lethal fenvalerate concentration in zebra fish
For the current biochemical study, freshwater zebrafish with 3 ± 0.21 cm in length and 0.31 ± 0.02 gm in weight were acquired. The selected fishes in glass tank were acclimatized for two weeks. The water change was done daily; fishes were fed with pellets. Exposure to sub-lethal (1/10th of 96th hour LC50) concentration of fenvalerate for one week was provided to the selected experimental group. After that control and experimental animals were subjected for biochemical assays [8].
Total protein estimation from muscle and gill
Determination of protein concentrations lies in the reactivity of the peptide nitrogen(s) under alkaline conditions with the copper (II) ions and the succeeding diminution of the folinciocalteau phosphomolybdic phosphotungstic acid to heteropolymolybdenum blue by the copper catalyzed oxidation of aromatic acids. The pH of the assay solution should be maintained at 10-10.5 because this method is sensitive to pH changes. A variety of compounds interfere include some amino acid derivatives, certain buffers, drugs, lipids, sugars, salts, nucleic acids and sulphydryl reagents by low concentrations of protein. These substances should be removed or diluted before running Lowry assay.
Lipid estimation from muscle and gill
Lipid content of the sample was extracted from 0.5 g gill and muscle of zebrafish was put thrice with chloroform: Methanol (2:1 v/v) according to Mahmoud, et al., 0.1 g muscle and gill tissue from the fresh fish were cut, rinsed with distilled water and dried to constant weight in a drying oven (60°C, 24 h). Dried samples were pulverized in a glass blender, homogenized with chloroform: Methanol mixture (2:1, v/v), vortex at 2800 rpm and filtered. The extract was shaken and equilibrated with about 20% of its volume to a saline solution. The extracted lipids were weighed and the percentage of lipid content was measured [9].
Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS- PAGE)
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) used for the separation of proteins based on their size. Charged molecule will migrate in an electric field towards an electrode with opposite signal. To determine the molecular weight of biological molecules, the mobility of a substance in the gel depends on both charge and size. To overcome this, the biological samples acquire uniform charge and the electrophoretic mobility depends primarily on size. Different protein molecules with different shapes and sizes needs to be denatured (with the aid of SDS) to lose their structural protein disparity. The negatively charged proteins with SDS, when loaded onto a gel placed in an electric field will migrate towards the anode (positively charged electrode) are separated by a molecular sieving effect based on size. The image by a staining (protein-specific) technique calculated by comparing its migration distance with that of a known molecular weight ladder (marker) offered the size of a protein [10].
Fenvalerate toxicity to zebrafish
In the present study fenvalerate toxicity for zebrafish was explored. In 20 μg/L concentration, 24th h exposure recorded 55% mortality. 82% mortality was obtained in 96th h of treatment. 62% mortality was registered after 24th h at 40 μg/L and further increased as 94% after 96 h of exposure. At 60 μg/L, 71% mortality was noted initially and it raised 99% at the 96th hour of the trial. In 80 μg/L the mortality rate ranged from 79% to 100%. Finally in 72 h and 96 h, all fishes were died and registered 100% mortality in 80 μg/L and 100 μg/L of fenvalerate concentration (Table 1).
| Fenvalerate concentration (µg/L) | Mortality (%) | |||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |
| 20 | 55 | 64 | 71 | 82 |
| 40 | 62 | 73 | 85 | 94 |
| 60 | 71 | 82 | 94 | 99 |
| 80 | 79 | 91 | 100 | 100 |
| 100 | 90 | 100 | 100 | 100 |
Table 1: Effect of different (20 µg/L-100 µg/L) concentrations of fenvalerate on zebrafish at various exposure times.
Biochemical changes due to fenvalerate toxicity
Total protein content of muscle and gill of zebrafish exposed to sub-lethal fenvalerate concentration (96 h): Total protein content of normal fish gill was 120 ± 1.22 mg/g and it reduced to 118.31 ± 0.5 mg/g, 117.4 ± 1.8 mg/g, 114.3 ± 2.1 mg/g and 110 ± 1.4 mg/g, after 24 h, 48 h, 72 h and 96 h respectively. The total protein content of the muscle also declined after 96 h treatment. In the control fish, total muscle protein was 80.2 ± 1.3 mg/g and it minimized to 78.4 ± 2.4 mg/g, 71.4 ± 2.2 mg/g in subsequent exposure of LC50. After 96 h of exposure of fenvalerate, muscle protein was 60 ± 2.7 mg/g in the zebrafish (Figure 3).
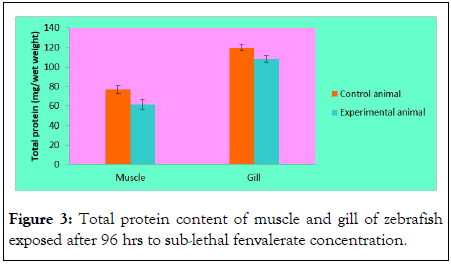
Figure 3: Total protein content of muscle and gill of zebrafish exposed after 96 hrs to sub-lethal fenvalerate concentration.
Lipid content of muscle and gill in sub-lethal fenvalerate concentration (96 h) exposure: In the present study, the muscle lipid content was 4 ± 0.46% wet weight (Figure 4). It has decreased considerably in muscle of fenvalerate exposed zebrafish (3.1 ± 0.12% wet weight) for 96 h. Compared with lipid from the muscle, the lipid content of gill was dramatically depleted from 6.5 ± 0.22% to 2.5 ± 0.31%.
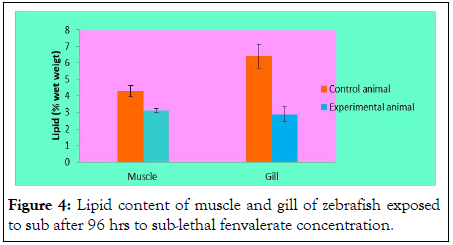
Figure 4: Lipid content of muscle and gill of zebrafish exposed to sub after 96 hrs to sub-lethal fenvalerate concentration.
SDS-PAGE analysis of protein from muscle and gill of zebrafish exposed to sub lethal fenvalerate concentration (96 h): Variations in protein bands between control and experimental group revealed the SDS polyacrylamide gel electrophoresis analysis. Figure 5 showed variations of gill and muscle protein of zebrafish exposed to fenvalerate. Fenvalerate inhibited the biosynthesis of proteins and induced the synthesis of stress proteins was the reason behind the less intensity in few sub units and appearance of few proteins in experimental samples [11].
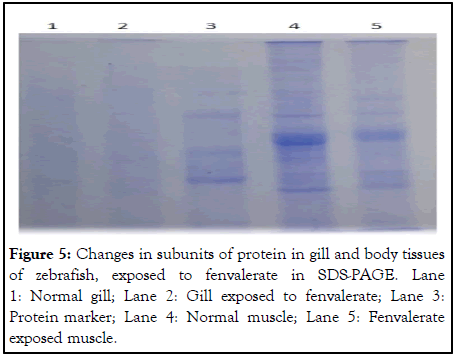
Figure 5: Changes in subunits of protein in gill and body tissues of zebrafish, exposed to fenvalerate in SDS-PAGE. Lane 1: Normal gill; Lane 2: Gill exposed to fenvalerate; Lane 3: Protein marker; Lane 4: Normal muscle; Lane 5: Fenvalerate exposed muscle.
The important objective of acute toxicity tests revealed stressful and pathological abnormalities leads to short death which evaluated the concentration of toxic materials obstructed their physiological tasks. The toxicity of agricultural pesticides is one of the main causes for drastic decline of fish population. Death is the chief decisive factor in toxicity experiments because of its obvious determination in ecological and biological significance [12]. In a biological system, the immediate pesticide toxic effect of an aquatic environment should be explicated. In the present study, the LC50 value for 96 h exposure of fenvalerate to zebrafish was 0.609. This value was almost 10 times lower than that of fishes such as, catla and rohu. In Cyprinus carpio the LC50 value for 48 h exposure to fenvalerate at pH 8.00 to 8.88 was reported to be 0.0210 mg/L.
LC50 study of Clarias batrachus reported the fenvalerate (freshwater catfish) content of 19 μg/L by Tripathi and Verma. The acute toxicity (LC50) in 96 h for catla fingerlings was 6 μg/L. Prusty, et al., reported 96 h LC50 value for Labeo rohita was reported as 5.36 μg/L. LC50 value of Channa punctatus at 96 h was reported as 2.13 μg L-1 in fenvalerate exposure. Coverage of fenvalerate reported detrimental to biochemical contents on Channa punctatus in swamps and Cirrhinus mrigala [13].
Normally fishes have less capability to metabolise and send out fenvalerate; thus they are vulnerable even at little concentration. When fish is exposed to toxicants, they readily utilize glycogen reserves for their survival. Water solubility of insecticides and lipophilicity was the key parameters which influence the uptake correlated with the toxicity of insecticides including fenvalerate. Stressed fishes also showed shrinkage in protein content and increase in total amino acids to meet the energy requirements. Uptake of insecticides through the gills, bloodstream causes fish toxicity [14].
In the present study, behavioural changes were observed when the zebrafish was fenvalerate exposure. Various behavioural changes were registered at sub-lethal and lethal fenvalerate concentration which includes movement of fishes to the bottom of the aquarium water. Also, noticed excess production of mucus substances from the gills and lead dashing the tank wall. These practical behavioural responses were noticed in dimethoate exposed common carp, Cyprinus carpio. Again, body colour did not change at lethal or sub-lethal concentration of fenvalerate. Santhakumar and Balaji found colour change in Anabas testudineus after the fish was exposed to monocrotophos and he reported imbalance in posture, erratic swimming and more surface activity, excess of mucus all over the body and sluggishness. At high concentration of fenvalerate toxicity, restless swimming, imbalances at surface to bottom and unusual surface activity was observed. The surface swimming habit is mainly to gulp maximum possible air to solve stress by the fishes [15].
In the present study increased opercular movements were observed which was similar to that of report in Clarias batrachus exposed to herboclin. The high rate of opercular movements in experimental animal may be due to heavy accumulation of mucous on gill tissues due to the toxicant. The changes in behaviour such as irregular swimming, surface swimming and mucous on the gills was noticed when zebrafish was exposed to fenvalerate indicated pollution of the habitat.
In this investigation fenvalerate induced biochemical changes in muscle and gills of zebra fish, total protein content depleted considerably in gills than in muscle tissue. In gill total protein depletion was about 24.4% after 96 h exposure than control whereas in muscle only 4% total protein content was depleted. Pesticides generally induce various enzymatic and biochemical changes in aquatic organisms especially in fishes [16-20].
Tiwari and Singh reported the effect of cypermethrin on Labeo rohita showed variations in biochemical composition in fish. In the structural point of view, proteins are vital and involve various biological activities among various types of cells. The decrease of protein level due to pesticide toxicity was reported previously by Mastan and Ramayana. Depletion of total protein content in present findings are in good agreement with previous findings of David, et al., they reported comparable results in Cyprinus carpio with cypermethrin exposure. Kumar and Gopal reported depleted level of total protein content in the liver of fish exposed with toxicants. In the present study, lipid content was decreased in the muscle of fenvalerate exposed zebrafish. Compared with muscle lipid, the lipid content of gill was dramatically depleted in the experimental animal. Fat content of zebrafish was significantly affected by fenvalerate; it was the basis for fat reduction in fishes. Gills are first target to pesticides. In Terapon jarbua, endosulfan affected the lipid content in Terapon jarbua. Vinodhini and Narayanan studied the total lipid profile with pesticide in the liver of common carp Cyprinus carpio L. Zutshi, et al., reported reduction of lipid content in Labeo rohita and, Hanan, et al., studied the impact of pesticide and reported reduced level of lipid in Clarias gariepinus.
In this study PAGE was used to analyze the protein profile of fenvalerate treated fish illustrated variations in protein profile between control and fenvalerate exposed animals. In the present study, fenvalerate exposed muscle and gill sample demonstrated least protein intensity in banding pattern compared to control group on PAGE. The variation in the subunit of muscle and gill protein may be manifested in the expression of some of the protein genes or production of stress proteins to survive at toxic conditions. The results of protein are in concurrence with Suneetha, et al., who analyzed changes in protein profile of Labeo rohita induced by fenvalerate. The mechanism of action of fenvalerate and other pesticides may significantly inhibit the expression of genes or may activate other set of genes leads to the production of mRNAs; afterwards which may be translated into stress induced proteins to survive in the stress conditions.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Beslin LG (2023) Toxicity Studies of Fenvalerate on Zebrafish, Danio rerio (Hamilton, 1822). J Clin Toxicol. 13:537.
Received: 13-Dec-2022, Manuscript No. JCT-22-20860; Editor assigned: 15-Dec-2022, Pre QC No. JCT-22-20860 (PQ); Reviewed: 29-Dec-2022, QC No. JCT-22-20860; Revised: 23-Mar-2023, Manuscript No. JCT-22-20860 (R); Published: 30-May-2023 , DOI: 10.35248/2161-0495.23.13.537
Copyright: © 2023 Beslin LG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.