
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review Article - (2020)Volume 10, Issue 2
Aortic coarctation (CoA) is not uncommon congenital cardiac disease. Surgical repair of native CoA is nowadays a common and safe procedure at early childhood. However, late complications, including re-CoA and aneurysm formation, are not uncommon. The incidence of these complications is dependent on the type of the initial operation. Both transcatheter and surgical repair play important roles in the treatment of late surgical complications. This article will review the incidence of late complications after CoA repair and will discuss the transcatheter treatment options for such complications in the view of recent advancement in transcatheter therapy.
Stents; Aortic Coarctation; Balloon angioplasty; Cardiac Catheterization; Descending aortic repair; Thoracic endovascular repair
CoA is the sixth most common congenital heart disease (CHD) accounting for 4–8% of all CHD and occurs in 4 out of 1,000 live births with a male predominance. CoA can occur as an isolated lesion, but is often associated with other cardiovascular lesions, such as bicuspid aortic valve (BAV) in 50–75% of the cases, aortic arch hypoplasia, subaortic stenosis, mitral valve abnormalities, ventricular and atrial septal defects and patent ductus arteriosus (PDA) [1,2]. The most important non-cardiac associated lesion is cerebral aneurysm present in up to 10% of patients, which is approximately 5 times higher than that in the general population [3,4]. It may cause isolated hypertension, aortic aneurysm or dissection, intracranial bleeding, coronary artery disease, congestive heart failure and endocarditis. Without treatment, the outcome for patients with CoA is poor. Historical data on the natural history of patients with CoA who survived beyond infancy showed a mean age of death of 34 years and 75% mortality by age 43 years. Therefore, most patients that require intervention undergo repair early in life or when initial diagnosis is made [5].
Surgical correction of CoA has been the standard treatment in infants and adolescents to prevent either the early or late complications related to the obstruction. The first successful surgical repair of CoA was performed by Crafoord [6]. The surgical repair technique quickly spread worldwide and was advanced by the introduction of diverse techniques. Survival after childhood repair is reported as 89% at 15 years and 83% at 25 years. Adults repaired between age 20 and 40 years have a 75% 25-year survival, whereas older patients have only a 50% 15- year survival [7]. Webb reported 72% to 82% 30-year survival overall [8]. Whatever the surgical technique, late post-repair complications are not uncommon, often decades later. The most important long-term complications include persistent systemic hypertension which has been reported to be 35%–68%, aortic re-CoA which has been reported to be 10% to 41%, aneurysm at the treated segment which has been reported to be 5% and 51% and pseudoaneurysm formation at the anastomotic site (around 10% of cases overall) [9-11].
In addition to conventional open repair, transcatheter therapy with balloon angioplasty (BA) and/or stenting emerged as treatment alternatives for older children and adults with discrete area of native CoA. Late complications such as re-CoA, aneurysm and pseudoaneurysm formation can occur after transcatheter therapy, which may need further interventions [12].
Types of initial repair
Since the first CoA repair done with end to end anastomosis; several modifications have been developed depending on the patient age and CoA anatomy (Figure1A-1F).
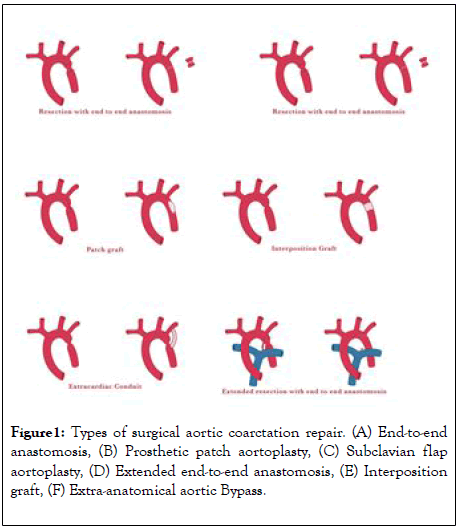
Figure 1: Types of surgical aortic coarctation repair. (A) End-to-end anastomosis, (B) Prosthetic patch aortoplasty, (C) Subclavian flap aortoplasty, (D) Extended end-to-end anastomosis, (E) Interposition graft, (F) Extra-anatomical aortic Bypass.
End-to-end anastomosis: This is the first surgical therapy documented for CoA, it was performed through a lateral thoracotomy [6,13]. The aorta is adequately mobilized and cross clamped proximal and distal to the CoA segment. Then the next step is to resect the CoA segment followed by a direct suture anastomosis of the remaining aortic arch and the descending aorta (end-to-end). The incidence of re-CoA is relatively high following this initial repair, with reported incidence rates of 41% to 51%. The occurrence of re-CoA is age-dependent and is highest if the surgery is performed in neonates. Given the high incidence of re-CoA, direct end-to-end anastomosis is not commonly used nowadays [14].
Prosthetic patch aortoplasty: Due to the high re-CoA rate following the end-to end anastomosis technique, physicians sought alternative surgical options to treat aortic CoA [15]. Patch augmentation, initially with Dacron, was used where the aorta was incised longitudinally through the CoA segment on the lateral wall of the aorta and a prosthetic patch is sutured across the incision which enlarges the vessel diameter. Although the usage of Dacron grafts resulted in a lower rate of re-CoA, a high incidence of aortic aneurysm formation was observed with this technique (20% to 40%) [16]. The usage of polytetrafluoroethylene reduced the rate of aneurysm formation to 7%, but led to a higher re-CoA rate (25%) instead [17]. For these reasons, patch aortoplasty has mostly been abandoned for the treatment of simple CoA. However, it is still being used for complex cases that require aortic arch reconstruction.
Subclavian flap aortoplasty: In this technique the subclavian artery (SCA) is ligated close to the origin of the left vertebral artery. The flap is generated by an incision of the SCA that is extended down onto the aortic isthmus and across the CoA segment. The flap is then folded downwards and sutured into the incised aorta, enlarging the previously stenosed aorta [18]. The re-CoA rate of this technique seems to be relatively low when performed in older children (0–3%) [19-20]. However, when applied to neonates, re-CoA may occur in up to 23% [21]. Although sacrificing the SCA does not result in left arm ischemia, it may cause claudication in the long term.
Extended end-to-end anastomosis: In contrast to a direct end-to-end anastomosis, the proximal clamp is placed across the aortic arch including the SCA or even the left carotid artery including the aortic arch. Distally, the aorta is clamped below the CoA segment. After ligation and division of the ductus arteriosus, the CoA segment is resected and the aortic arch is opened on its inferior aspect, followed by end-to-end anastomosis of the opened arch and the descending aorta [22]. The procedure can be performed with low peri-operative mortality and reports show relatively low re-CoA rates of 4% to 13% [23-27].
Interposition graft: This technique has been reserved for patients in whom outgrowth of the graft is not a concern, or in patients with long-segment CoA. After the aorta is cross-clamped and the CoA segment resected, a tube graft of either aortic homograft or Dacron is sewn into the aorta, creating an unobstructed path for blood. The main disadvantage of this technique is that it requires a longer cross-clamp time for two surgical anastomoses to be sewn, and the tube graft will not grow with the patient [28]. The short- and long-term results in adults are respectable: Yousif and colleagues reported a peri-operative mortality of 0% and no re-CoA events during mean follow-up of 10 ± 7.6 years [29]. Comparable outcome has been reported by other groups [30].
Extra-anatomical aortic Bypass: This technique aims to provide additional blood flow to the distal aorta, however leaves the CoA segment in situ. This is generally performed via a median sternotomy, with cardiopulmonary bypass support [31,32]. Proximally, a prosthetic conduit is anastomosed to the ascending aorta or the SCA and is anastomosed to the descending aorta distally, thereby bypassing the CoA [33]. This technique is especially useful when concomitant cardiac procedures (such as aortic valve replacement or coronary artery bypass grafting) need to be performed as well [34].
In the most recent 2018 AHA/ACC Guideline for the management of adults with congenital heart disease; the significant re-CoA was class I for re-intervention (Surgical repair or catheter-based stenting). Significant re-CoA has been defined as follows: upper extremity/lower extremity resting peak-to-peak gradient >20 mm Hg or mean Doppler systolic gradient >20 mm Hg; upper extremity/lower extremity gradient >10 mm Hg or mean Doppler gradient >10 mm Hg plus either decreased left ventricular systolic function or aortic regurgitation; upper extremity/lower extremity gradient >10 mm Hg or mean Doppler gradient >10 mm Hg with collateral flow. This should be coupled with anatomic evidence for CoA, typically defined by advanced imaging (Cardiac MRI or Cardiac CT). In the same guideline, BA for adults with re-CoA was class IIb and may be considered if stent placement is not feasible and surgical intervention is not an option [35]. Besides re-CoA, aneurysm formation is another complication after CoA repair that may require reintervention. The aneurysm usually develops at the site of the previous repair, but occasionally involves the ascending aorta, especially in the setting of BAV. The societal guidelines for CoA do not clearly define indications for the treatment of aneurysm formation after CoA repair; however, one may refer to the guidelines for the management and treatment of thoracic aortic aneurysms [36,37]. For aortic arch aneurysms, surgical intervention is recommended at an aortic diameter of 55 mm or more. For descending aortic aneurysms, intervention is recommended for aortic diameter of 55 mm or more if treatment with transcatheter modalities is feasible. If transcatheter treatment is not possible, open surgical repair is indicated in patients with an aortic diameter of 60 mm or more. Whenever the aneurysm progress to aortic dissection and/or pseudo-aneurysm formation the mortality increases exponentially and immediate intervention with transatheter therapy or surgery is mandatory [38].
Given high late complication rates necessitating reintervention in 5% to 50% of cases after repair, and the high mortality rate in redo repair compared to native CoA repair that ranges from 1% to 3%, but can be as high as 5% to 10% for patients with significant comorbidities such as significant coronary lesions, left ventricular dysfunction and peripheral vascular disease; the transcatheter therapy emerged as an attractive and less invasive alternative to surgery with good success rate and low mortality <1% [39].
Balloon angioplasty
BA has a high rate of re-CoA when used as the primary modality to repair a native CoA; However, the trend toward re-CoA is less when it is used to treat aortic re- CoA [39]. Multiple studies have demonstrated the sound outcome of BA for re-CoA [40,41]. The reported procedural success rate ranges from 80% to nearly 100% (42). Freedom from re-CoA after BA is quite variable, with reported rates of 6% to 53% [43]. The scar tissue at the site of the previous repair has a lower tendency for vascular remodeling, thus leading to a lower rate of aneurysmal dilation after BA. Aortic wall injury occurs in approximately 1% to 2% of cases [42]. In young children, with a significant amount of aortic growth yet to take place, BA is the preferred treatment modality for re-CoA. BA may be also considered if stent placement is not feasible and surgical intervention is not an option.
Stenting for re-CoA
Stenting is considered advantageous when compared to BA alone; it improves luminal diameter, results in minimal residual PPG, and sustained hemodynamic benefit. It also can tack intimal flaps to the aortic wall potentially allowing healing to occur this way reducing the risk of dissection and aneurysm formation. Furthermore, stent implantation does prevent vascular recoil resulting in re-CoA [39]. Nowadays, BA with simultaneous stenting is considered to be the preferred treatment option for adolescent and adult patients with native or re-CoA. Since the first cases of CoA stenting were reported in the 1990s, the encouraging outcome of this procedure has been described in considerable numbers of individual series reporting both short-term and intermediate-term results [44,45]. In 2007, a large multi-center retrospective series was reported by the Congenital Cardiovascular Interventional Study Consortium (CCISC) on 565 procedures of stent implantation performed in 555 patients with CoA and re-CoA between 1989 and 2005 [46,47]. The success rate of reducing PPG to less than 20 mmHg was 98%. There was a mean ± standard deviation (SD) reduction of the PPG from 31.6 ± 16 to 2.7 ± 4.2 mmHg and an increase in diameter from 7.4 ± 3.0 to 14.3 ± 3.2 mm. Similar procedural success rate was reported recently for both native and re-CoA by Hartman et al. (1,612 patients) and Yang et al. (561 patients) [48,49]. Recently, the results of the Coarctation of the Aorta Stent Trial (COAST) trial, a study that aimed to assess the safety and efficacy of CP stents in children and adults with native or re-CoA were published [50]. During the two-year follow-up no deaths, serious adverse events or surgical interventions were reported. All patients experienced satisfactory post procedural results with low rates of complications including aneurysms (5.7%) and stent fracture (11% at 2 year) without loss of stent integrity, stent embolization, aortic wall injury, or re-CoA. Re-interventions (8.7%) that occurred during the two-year follow-up period were for stent re-dilatation in order to account for the patients' somatic growth and address of aneurysms. In the review by Hartman et al. the rate of aneurysms was 1.5%, stent fracture 1.6%, and reintervention 11%. Relatively similar findings were obtained by Yang et al. in his meta-analysis. It has been proposed that the use of covered stents reduces the risk of aneurysms, dissection, and rupture. The recently published, COAST II trial which is a multi-center, single arm trial using the covered CP stent for the treatment and/or prevention of Acute Wall Injury (AWI) in native or re-CoA associated with one or more of the following: acute or chronic aortic wall injury, nearly atretic descending aorta to 3 mm or less in diameter, genetic syndromes associated with aortic wall weakening (e.g., Marfan syndrome, Turner syndrome, familial BAV with ascending aortic aneurysm), or advanced age (60 years or older). A total of 158 patients were involved. The PPG decreased from 27 mmHg to 4 mmHg. The overall success rate was 92%. There was no AWI, repeat intervention or death [51]. However, in a randomized trial of 120 patients with severe native CoA, there was no difference in the rate of re-CoA and pseudoaneurysm formation after 31 months of follow-up between patients who underwent implantation using a bare metal stent and those with a covered stent and this result was similar to what previously has been published in case reports and small series [52]. Nevertheless, covered stents offer the advantage of excluding any stretchinduced wall trauma from the endoluminal aspect of the aorta, particularly in the catastrophic event of aortic rupture as revealed by COAST II trial.
Technical aspects
The type of re-CoA is primarily dependent on the initial surgical approach. Other contributing factor is the presence of isthmus hypoplasia. In patients with direct end-to-end anastomosis surgery; the re-CoA tends to be focal (Figure 2) comparing to extended end-to-end anastomosis and subclavian flap aortoplasty (Figure 3). This is probably related to resection and repair/ anastomosis of larger segment and it is related wide sutures line that heal with fibrosis.
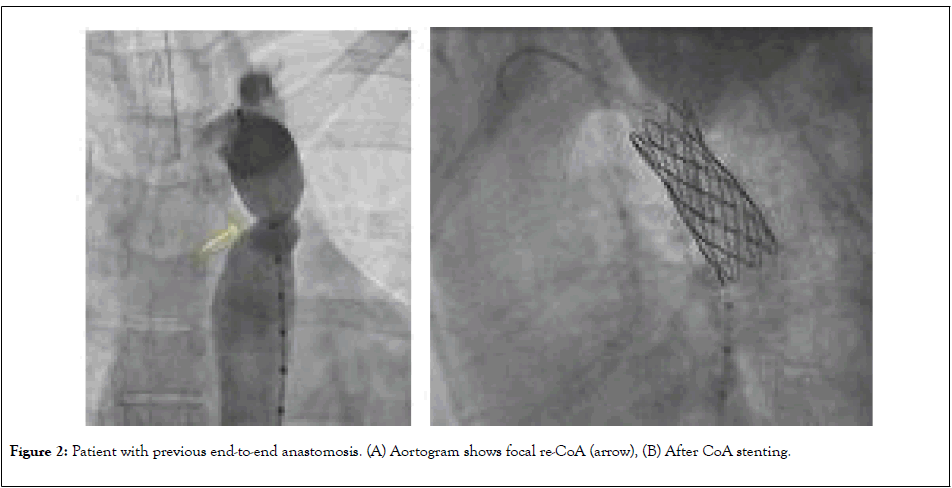
Figure 2: Patient with previous end-to-end anastomosis. (A) Aortogram shows focal re-CoA (arrow), (B) After CoA stenting.
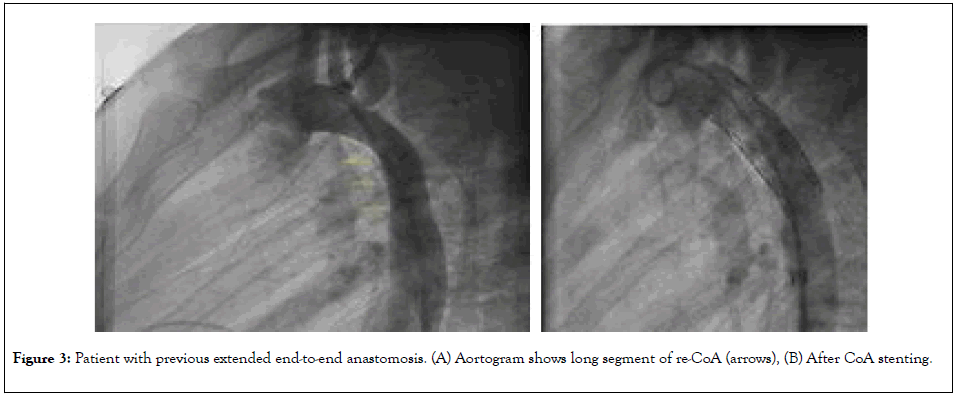
Figure 3: Patient with previous extended end-to-end anastomosis. (A) Aortogram shows long segment of re-CoA (arrows), (B) After CoA stenting.
Stenting for aneurysm/pseudoaneurysm
Aneurysms in the region of the previous repair and aneurysms remote from the repair carry a life-time risk of rupture. The frequency of late aneurysms depends on the surgical era, the patient’s age at operation, the postoperative interval, and the surgical technique employed [53]. It has been also suggested that an aneurysm may occur if all the pathological tissue is not resected during open surgical repair. Recent studies show the overall incidence of postoperative aneurysms to be 5% to 9%, with the lowest incidence reported after end-to-end anastomosis or after extraanatomic tube grafts and higher incidence with patch aortoplasty [54]. The average time to aneurysm formation has been reported to be up to 12 years after initial repair with a progressive increase in prevalence over time [55].Emergency presentation is not uncommon with rupture associated with 7% mortality (Figure 4) [56]. Surgical repair carries higher mortality rate that ranges from 1% to 3%, but can be as high as 5% to 10% for patients with significant comorbidities [45]. Thoracic endovascular aortic repair (TEVAR) emerged as safe and effective alternative to surgery. Reports and studies have shown a procedural success rate of up to 100% (Figure 5) [57-59]. Potential complications are similar to those caused by BA or stent placement for re-CoA as described earlier. Endoleaks can occur due to suboptimal endograft apposition to the aortic wall, especially in cases of challenging anatomy. Another devastating complication that can occur in up to 10% of cases after stent graft implantation is spinal cord ischemia, leading to paraparesis or paraplegia [60].
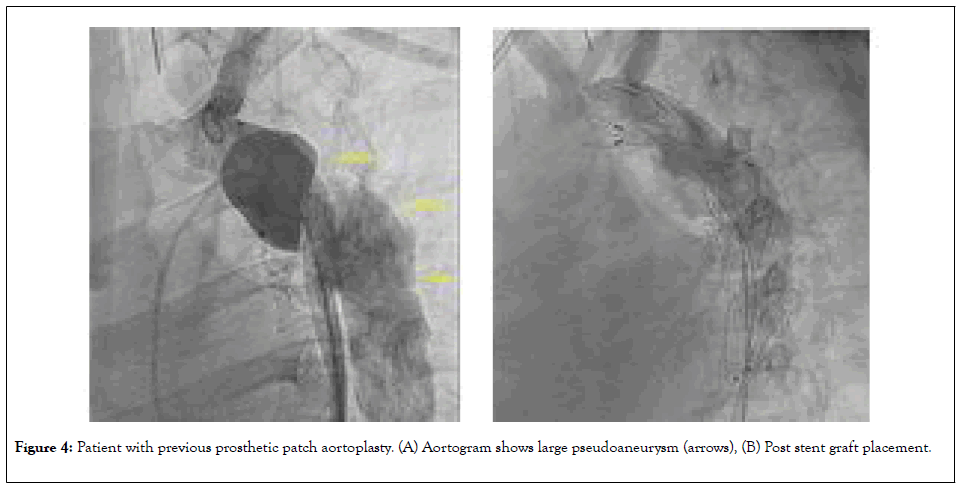
Figure 4: Patient with previous prosthetic patch aortoplasty. (A) Aortogram shows large pseudoaneurysm (arrows), (B) Post stent graft placement.
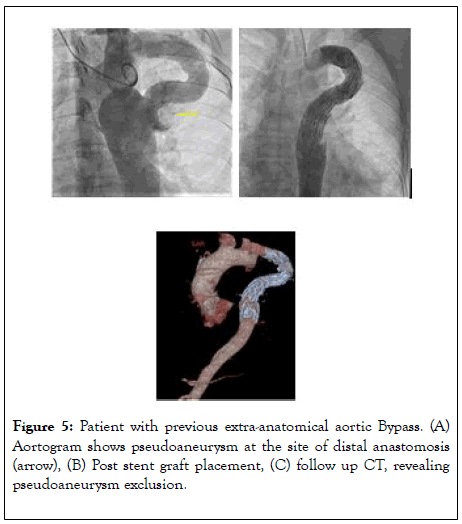
Figure 5: Patient with previous extra-anatomical aortic Bypass. (A) Aortogram shows pseudoaneurysm at the site of distal anastomosis (arrow), (B) Post stent graft placement, (C) follow up CT, revealing pseudoaneurysm exclusion.
When transcatheter therapy is not successful or applicable, surgical repair remains the only treatment option left. This may include re-CoA involving the transverse arch or other anatomic variants not amenable to stent grafting or complications of stent grafting including uncontrollable endoleak or graft infection. The surgical repair depend upon the re-CoA type. Focal re-CoA may be undertaken via left thoracotomy, whereas more complex re-CoA involving large segment that may have concomitant aneurysmal disease or hypoplastic aortic arch may require open surgical repair. Redo surgery for aortic aneurysm not amenable for transcatheter therapy is challenging with a significant mortality (up to 15%) and morbidity rate (up to 45%) and should be done in highly specialized centre with high volume [61].
CoA is not uncommon and it is lifelong serious disease. Early intervention is associated with better outcomes. Surgical intervention at early childhoods is associated with various medical and surgical complications at long term follow up. Timely fashioned clinical follow up to detect such complications early and to be treated promptly before devastating complications such as aortic dissection and rupture occur. New advances in transcatheter therapy proved to be safe and effective and decrease the need for redo surgery and it is associated morbidity and mortality.
Special acknowledge to Melissa G Beiruty, king Abdulaziz University Hospital
Citation: Alkashkari W, Albugami S, Althobaiti M, Alfouti M, Alrahimi J, Kinsara A, Alzahrani A, Fernandez JA, Tash A, Aburemish H (2020) Transcatheter intervention for late complications after aortic coarctation surgical repair. J Clin Trials 10:402. doi: 10.35248/2167-0870.20.10.402
Received: 04-Mar-2020 Accepted: 12-Mar-2020 Published: 19-Mar-2020 , DOI: 10.35248/2167-0870.20.10.402
Copyright: © 2020 Alkashkari W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.