Journal of Depression and Anxiety
Open Access
ISSN: 2167-1044
ISSN: 2167-1044
Case Report - (2014) Volume 3, Issue 3
Background: Panic disorder (PD) is characterized primarily by the presence of recurrent and unexpected panic attacks, followed by at least one month of persistent concern about other attacks, the possible consequences of attacks and a significant behavioral change related to the attacks. The neurocircuitry of fear includes two pathways for processing of sensory information. Transcranial direct current stimulation (tDCS) use has not been reported for PD.
Objective: To report the results of an experimental tDCS protocol for ameliorating anxiety symptoms in a patient with panic disorder.
Method: The current report is based on a single case study. We used an experimental tDCS intervention protocol as to inhibit the right dorsolateralprefrontal cortex during a period of two weeks. Symptoms were assessed by adequate clinical scales.
Results: We hereby describe a 44-year-old woman with PD who successfully underwent a tDCS intervention, with important ameliorating of her symptoms.
Discussion and Conclusion: To the best of our knowledge, this it is the first report using tDCS for PD. Some study limitations, however, should be addressed. Our findings are based on a case study, thus having limited generalizability. Nonetheless, these encouraging results should be seen as hypothesis-driving for further controlled, randomized trials exploring the impact of tDCS in the treatment of PD and maybe other anxiety disorders.
Keywords: tDCS; Neuromodulation; Panic disorder
Panic disorder (PD) is characterized primarily by the presence of recurrent and unexpected panic attacks, followed by at least one month of persistent concern about other attacks, the possible consequences of attacks and a significant behavioral change related to the attacks. For a diagnosis of PD, the panic attacks cannot be better accounted for by another mental disorder, by physiological effects resulting from the use of substances or by other medical conditions, such as hypothyroidism [1]. PD has a 5% lifetime prevalence and 1% annually [2]. Current treatment strategies have been based on both psychological and pharmacological therapies, although treatment-resistance and low adherence due to adverse effects are some issues that compromise optimal treatment.
The neurocircuitry of fear includes two pathways for processing of sensory information. The shorter path consists of the rapid spread of autonomic and behavioral responses in potentially hazardous situations. In this case, the major regions involved are the anterior thalamus and the central and lateral regions of the amygdala. The latter is, in particular, the coordinator of this process because it triggers regions that themselves emit responses. In the longer path, the information passes through several regions, including the cortex, which allows for a more refined analysis of inputs. In the case of PD, the hypothesis is that the neurocircuitry would result in dysregulated activation and coordination with increased subcortical activity and consequent neuroendocrine activation, both behavioral and autonomic [3,4].
Regarding new interventional strategies, the development of non-invasive brain stimulation techniques, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), has shown promising results [5,6]. tDCS is based on the application of a weak, direct electric current delivered over the scalp as to induce polarity-dependent changes in cortical excitability – respectively, anodal and cathodal stimulation induces increasing and decreasing of cortical excitability [7,8]. When applied daily, neuromodulation techniques such display clinical effects and has been successfully used for the treatment of psychiatric disorders [9-11]. However, tDCS use has not been reported for PD. We hereby describe a 44-year-old woman with PD who successfully underwent a tDCS intervention, with important ameliorating of her symptoms.
“Ms. S.” developed panic attacks progressively more frequent during the past 3 years despite adequate treatment protocols with several medicines in adequate doses. In fact the patient had already been treated, in cronological order, with venlafaxine (up to 300 mg/day for 8 weeks), sertraline (up to 200 mg/day for 6 weeks), amitriptiline (as augumentarion strategy with sertraline for 3 weeks) and quetiapine in monotherapy (up to 400 mg/day for 6 weeks). She presented no significant improvement of her symptoms, au countraire, important adverse effects occured (mainly gastrointestinal and sleep disturbances) that ultimately lead her to discontinue the use of pharmacotherapy.
Considering the severity of her symptoms and the failure of other therapies, tDCS was started after a written informed consent was provided. We performed 10 consecutive daily tDCS sessions (except for weekends as stated below). The patient presented limitation of her independecy for daily activities due to severity of panic attacks. In fact, she was unable to continue working and other activities rather than routine were markedly diminuished. Anxiety symptoms substantially improved during the 10-day treatment course. At baseline, the patient did not present with psychiatric comorbidity such as depression or cognitive impairement. After one month of treatment, the patient was asymptomatic and reported significant clinical gains (Figure 1). There were no significant clinical or cognitive side effects, except for a mild transient local eritema under the electrode site.
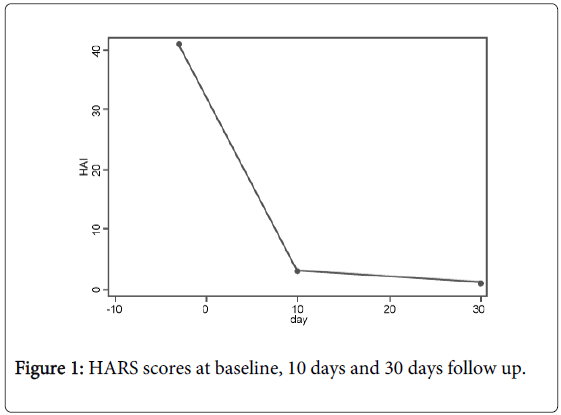
Figure 1: HARS scores at baseline, 10 days and 30 days follow up.
Procedure and assessment
The cathode was positioned over the right dorsolateral prefrontal cortex (DLPFC) and the anode was placed extracephalic over the contralateral deltoid. We used a direct current of 2.0 mA for 30 minutes per day. The 25 cm2-rubber electrodes were wrapped in cotton material, which was moistened with saline as to reduce impedance. For assessment of anxiety symptoms we used the Hamilton Anxiety Rating Scale (HARS). We also assessed depressive symptoms through the Hamilton Depression Rating Scale (HDRS) - version 17 itens; and cognitive functions with the Montreal cognitive Assessment (MOCA).
The use of cathodal stimulation over the right DLPFC was based in recent studies. Pallanti et al. [12] reviewed the use of repetitive transcranial magnetic stimulation (TMS) over the DLPFC for the treatment of anxiety disorders. Although diverse protocols were used, most trials using low-frequency rTMS (which similarly to cathodal tDCS decreases local cortical excitability) displayed positive results [6]. Specifically for PD, in an interesting open-label trial with three patients, the subjects who were enrolled had a history of the disease for at least one year and they had unsuccessfully followed psychotherapy and pharmacological treatment. The patients received 10 sessions during two weeks; each session lasted 30 trains of 60 seconds at a frequency of 1 Hz, on the right dorsolateral prefrontal cortex, at 110% of the motor threshold. All three patients experienced a modest and partial symptom improvement that did not seemed to be clinically relevant. Two patients accepted to participate in a TMS second phase, where the previous stimulation parameters were alternated with an application of 30 trains of 20 Hz during 2 seconds on the left prefrontal cortex. This alternate application of high and low frequency TMS in each session was also well tolerated, but failed to produce additional improvement [13].
Recently Mantovani et al. (2007) used rTMS as to inhibit the right DLPFC for 2 weeks in an open-label trial with satisfactory clinical response [14]. The same group in a further randomized controlled trial replicate the intervention protocol for twenty-five patients. Results demonstrated significantly better improvement in panic symptoms with active compared with sham rTMS. Clinical improvement was sustained at 6-month follow-up [15]. Moreover, regarding tDCS studies, Shiozawa et al. used a similar cathodic tDCS protocol for treating a patient with generalized anxiety disorder with satisfactory clinical results[16].
In the present case study, we performed cathodal stimulation targeting the right DLPFC, which theoretically might have diminished neuronal activity in such area, secondarily modulating other cortical and subcortical structures involved in PD pathophysiology such as the medial prefrontal cortex, the amygdala and the insula. Importantly, by anode positioning over the contralateral deltoid we were able to assert that anxiety improvement was not primarily related to the increase in cortical activity induced by tDCS. This montage might have also contributed for modulating deeper cortical and subcortical structures. Nonetheless, it is possible that the left DLPFC was secondarily modulated due to the decrease in activity of the right DLPFC.
To the best of our knowledge, this it is the first report using tDCS for PD. Some study limitations, however, should be addressed. Our findings are based on a case study, thus having limited generalizability. The lack of a control group should also add some limitation to the results as clinical improvement could have been magnified augmented by placebo effect. Nonetheless, these encouraging results should be seen as hypothesis-driving for further controlled, randomized trials exploring the impact of tDCS in the treatment of PD and maybe other anxiety disorders.