Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Research Article - (2014) Volume 1, Issue 1
The objective of this research was to study the permeation of lipophilic drug lamotrigine (LTG) using terpenes as permeation enhancers. Transdermal patches were formulated using one of the permeation enhancers namely Nerolidol, Limonene, Linalool, Carvone, Fenchone, Menthol, Geraniol, Farnesol. LTG patches were prepared by solvent casting method. The prepared patches were evaluated for drug content, thickness, and weight variation, folding endurance, moisture uptake, water vapour transmission, in-vitro diffusion study, ex-vivo permeation study and skin irritation study. Fourier Transform Infrared study revealed no interaction among the drug, polymers and terpenes used in the present study. The in vitro drug release studies were performed in 7.4 pH phosphate buffer using a Franz diffusion cell. Different formulations were prepared and variations in drug release profiles were observed. The cumulative percentage drug release of different terpenes was found to be in the order - limonene > fenchone > linalool > menthol > geraniol > carvone > nerolidol> farnesol. From the “n” values of optimized formulations which ranged from 0.6 to 0.7, it is evident that the release mechanism follows non-fickian type of diffusion which might be due to a combination of lipophilic and hydrophilic polymers used. Enhancement in drug release and ex-vivo skin penetration of LTG was found to depend on nature and concentration of terpenes and polymers. The skin irritation test was performed in rabbits and these results suggested that both placebo and drug-loaded films produced negligible erythema and edema compared to 1% sodium lauryl sulfate solution as the standard irritant. Formulations LLH3Lm (2.5%), LLH3Lm (5%) with Eudragit RL100, HPMC E15LV at 2.5% and 5% limonene concentration were found to show optimum drug release, improved permeability, steady state transdermal flux and reduced lag time (P<0.001) when compared to control formulation.
Keywords: Epilepsy; Eudragit; Limonene; Skin
Lamotrigine (LTG) is an anticonvulsant drug used in the treatment of epilepsy and bipolar disorder. Existing approved formulations of lamotrigine present cyclical plasma concentration of drug with peaks occurring after administration followed by troughs occurring before the next administration. In particular for the treatment of epilepsy, it is speculated that the troughs may lead to breakthrough seizures and may result in some adverse events. In special population like pregnant women, bioavailability of LTG is reduced by 50% due to its increased clearance attributed to increased renal blood flow and estradiol induced glucoronidation of LTG [1]. These problems can be overcome by bypassing the first pass metabolism and sustaining the drug release, both of which can be achieved by formulating drug in a transdermal system.
The present research work is hence aimed at formulating LTG, a class II drug with log P value of 2.5 as transdermal patch as it meets the criteria [2] a drug molecule should possess for it to be formulated as transdermal system. Transdermal patches were prepared by solvent casting technique using permeation enhancers [3] such as solid and liquid terpenes which varied in log P value and boiling point. Permeation enhancers promote skin permeability by altering the skin as a barrier to the flux of a desired penetrant. Shelke et al., studied the diffusion characteristics of indomethacin from castor oil based polyurethane class of penetration enhancers. Slow and prolonged release of drug was reported in the absence of penetration enhancers thereby demonstrating their role in drug release [4]. Raghavendra et al. formulated matrix system of atenolol in a graft copolymer of acrylamide and xanthan gum [5]. In another study [6], transparent, smooth and flexible zidovudine transdermal patches were formulated using Tween 80 as penetration enhancer. Though different classes of penetration enhancers are being used, their efficiency is limited by adverse reactions like toxicity and skin irritation [7]. Terpenes, the most advanced class of penetration enhancers derived from natural sources in this context offer several advantages over the synthetic counterparts. Terpenes are reported to be safe and effective on the skin [8,9] and are listed as “Generally Regarded As Safe” (GRAS). Hence it was aimed to formulate LTG transdermal patches using this class of penetration enhancers and to study the influence of terpenes on the transdermal permeation of LTG.
Materials
Lamotrigine (purity 99.9%) was obtained from RA chem limited, Hyderabad. Terpenes were obtained from Alfa Aesar Johnson Matthey Chemicals India Pvt. Ltd, Hyderabad. Propylene glycol, Di butyl phthalate, Glycerin and Ethyl cellulose were purchased from SD Fine chemicals, Mumbai. HPMC E15LV was purchased from Yarrow chem. Products, Mumbai. Poly vinyl alcohol (PVA) was obtained from Shinetsu, Japan and PVP K-30 from Universal Products Manufacturing Ltd, England. Eudragit RS 100, Eudragit RL 100 were obtained as gift samples from Evonik roehm pharma polymers, India. All other chemicals were of analytical grade.
Methods
Preparation of transdermal patches: Placebo patches, drug loaded patches with and without permeation enhancers were prepared by solvent casting method. Teflon petri plates of internal diameter 6 cm, were used for casting films of different polymer ratios (Eudragits, PVA, PVP, HPMC and Ethyl Cellulose) as given in Table 1. Teflon petri dishes were used as they give smooth and even films. Specified quantities of the polymers were dissolved in the solvents by soaking them for 1-2 h. Specified quantity of drug was dissolved in small amount of the solvent used for polymeric solution and drug solution was added to the polymeric solution under stirring. Plasticizer and penetration enhancers i.e., nerolidol, linalool, carvone, farnesol, limonene, geraniol, fenchone, menthol at 2.5% and 5% concentration was incorporated into this solution, sonicated for 2 min and then the solution was poured into teflon petri dishes. Different plasticizers like glycerin, Di-butyl phthalate (DBP) and propylene glycol were employed. The plate was kept at room temperature for 24h. An inverted funnel was placed over the plate to control the rate of drying.
| Formulation Code | Eudragit RL100 (%) | Eudragit RS100 (%) | HPMC E15LV (%) | PVA (%) | PVP K30 (%) | Ethyl Cellulose (%) | DBP (30%) (ml) | Propylene glycol (15%) (ml) | Glycerol (50%) (ml) | Solvent System |
|---|---|---|---|---|---|---|---|---|---|---|
| LPA1 | -- | -- | -- | 1 | 4 | -- | -- | -- | 0.23 | Water |
| LPA2 | -- | -- | -- | 2 | 3 | -- | -- | -- | 0.23 | Water |
| LPA3 | -- | -- | -- | 3 | 2 | -- | -- | -- | 0.23 | Water |
| LPE1 | -- | -- | -- | -- | 4 | 1 | 0.23 | -- | -- | Chloroform |
| LPE2 | -- | -- | -- | -- | 3 | 2 | 0.23 | -- | -- | Chloroform |
| LPE3 | -- | -- | -- | -- | 2 | 3 | 0.23 | -- | -- | Chloroform |
| LLS1 | 8 | 2 | -- | -- | -- | -- | 0.23 | -- | -- | DCM:Et |
| LLS2 | 6 | 4 | -- | -- | -- | -- | 0.23 | -- | -- | DCM:Et |
| LLS3 | 4 | 6 | -- | -- | -- | -- | 0.23 | -- | -- | DCM:Et |
| LSH1 | -- | 4 | 1 | -- | -- | -- | -- | 0.07 | -- | DCM:Et |
| LSH2 | -- | 3 | 2 | -- | -- | -- | -- | 0.07 | -- | DCM:Et |
| LSH3 | -- | 2 | 3 | -- | -- | -- | -- | 0.07 | -- | DCM:Et |
| LLH1 | 4 | -- | 1 | -- | -- | -- | -- | 0.07 | -- | DCM:Et |
| LLH2 | 3 | -- | 2 | -- | -- | -- | -- | 0.07 | -- | DCM:Et |
| LLH3 | 2 | -- | 3 | -- | -- | -- | -- | 0.07 | -- | DCM:Et |
Table 1: Formulation of transdermal patches without permeation enhancers.
L: Lamotrigine; P: PVP; A: PVA; L: Eudragit RL 100; S: Eudragit RS 100; H1-3: HPMC E15LV; DCM: Dichloromethane; Et: Ethanol
Optimized formulations from Table 1were used for further studies to study the influence of permeation enhancers.
Fourier transform infrared (FTIR) spectroscopy: FTIR spectra of pure lamotrigine and the optimized formulation were recorded with a FTIR spectrophotometer (FTIR-Shimadzu 8400 S, Japan) frsom 4000- 400 cm1 using KBr pellets. The pellets were made by applying a pressure of 100 kg cm2 to a mixture of formulation components and KBr (1:20) for 10 min in a hydraulic press (KP, Kimaya Engineers, India).
Evaluation of transdermal patches
a. Drug content: The patches were cut and dissolved in 5ml of methanol and made up to 100ml using phosphate buffer saline pH 7.4. The volumetric flask was kept for bath sonication for 30 min for mixing. The solution was passed through the whatmann’s filter paper, diluted appropriately and the drug content was measured spectrophotometrically against corresponding placebo patch at 305.6 nm [10].
b. Thickness variation test: The thickness of the films was measured at three different points using micrometer screw gauge and mean values were calculated [11,12].
c. Weight variation test The formulated films were prepared in triplicate. Three films from each batch were weighed individually and the average weight was calculated [13].
d. Folding endurance
Folding endurance of patches was determined by repeatedly folding a small strip of film at the same place till it broke. The number of times, the film could be folded at the same place without breaking gave the value of folding endurance [14].
Moisture uptake the films (n=3) were weighed accurately and placed in a desiccator chamber where a humidity condition of 75% RH was maintained by using saturated solution of sodium chloride. The films were taken out after 3 days and reweighed. The percentage of moisture uptake was calculated [15].
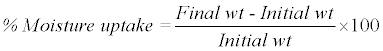
e. Water Vapor Transmission Rate (WVTR) studies Glass vials of equal diameter were used as transmission cells. The transmission cells were washed thoroughly and dried in oven at 100°C for some time. About 1g anhydrous calcium chloride was placed in the cells and respective polymer film (1sqcm) was fixed over brim. The cells were accurately weighed and kept in a closed desiccator containing saturated solution of potassium chloride (200 ml) to maintain a relative humidity of 84%. The cells were taken out after 24h and weighed after storage. The amount of water vapor transmitted was found using following formula [16].
WVT=WL/S
Where,
W = water vapor transmitted in gm,
L = thickness of the film in cm,
S = exposed surface area in square cm.
It is expressed as the number of grams of moisture gained/h/cm2.
f.: In vitro diffusion studies: Franz diffusion cell with receptor volume of 25 ml was used for diffusion studies. Dialysis membrane soaked in 7.4 pH phosphate buffer for 24 hours was mounted between the donor and receptor compartments. Patch formulation (3 cm×3 cm) was applied uniformly on the dialysis membrane and the compartment clamped together. The receptor compartment was filled with phosphate buffer saline pH 7.4 and the hydrodynamics in the receptor compartment was maintained by stirring with a magnetic bead. At predetermined time intervals (0, 1, 2, 4, 6, 8 and 12 h), 1 ml of sample was withdrawn and an equal volume of buffer was replaced. The samples were analyzed spectrophotometrically after appropriate dilution for drug content. The experiment was done in triplicates.
g. Ex-vivo skin permeation studies for optimized formulations: The skin isolated from wistar rats by reported procedure [10] was mounted onto a franz diffusion cell in such a way that the dermis side was in constant contact with the receptor solution. The receptor compartment was filled with phosphate buffer saline pH 7.4. The stratum corneum was facing the donor compartment and patch was placed on this, and the hydrodynamics in the receptor compartment was maintained by stirring with a magnetic bead. At pre-determined time intervals (0, 1, 2, 4, 6, 8 and 12 h), 1ml of sample was withdrawn and an equal volume of buffer was replaced. The samples were analyzed after appropriate dilution for drug content spectrophotometrically.
Ex vivo permeation rate studies such as percentage drug release, steady state transdermal flux (SSTF), permeability coefficient, lag time and enhancement ratio for percutaneous absorption of lamotrigine using terpenes across rat skin were estimated for different formulations. One way ANOVA by Tukey’s multiple comparison test for these parameters were carried out using Graph Pad. Tukey’s multiple comparison test is used to find means that are significantly different from each other.
h. Skin irritation studies: After approval from Institutional Ethics Committee with Id no: GPRCP/ IACE/ 11/13/3/ PCE/ AE-7, the study was conducted as per the protocol. A primary skin irritation test was carried out on an unbraided skin of two healthy rabbits weighing between 1.5-2 kg. The unbraided skin was cleaned with rectified spirit for placing the patches. The standard irritant (1% sodium lauryl sulfate solution), control patch, placebo, test (patch with drug and permeation enhancers) were placed on the left and right dorsal surface of the same rabbit and the other rabbit was kept as control. The patches were removed and the skin was examined for erythema/edema after 24, 48 and 72 h. The score was given according to the Primary Dermal Irritation Index classification (PDDI).
i. Stability studies: The selected formulations were packed in amber colored bottles, which were tightly plugged with cotton and capped with aluminum. They were then stored at room temperature for one month and evaluated for their appearance, drug content and folding endurance.
Fourier Transform Infrared (FTIR) spectroscopy
Drug-excipient compatibility in optimized formulation was evaluated by FTIR analysis. FTIR spectrum is displayed in Figure 1. The principal peaks of lamotrigine were observed at wave numbers of 1052.17, 1292.49 cm1 correspond to C-N stretch; 1619.74 cm1 to N-H bending; 3317.15 and 3211.38 cm1 to N-H stretching; 756.64 cm1 to C-Cl stretching. The characteristic peaks of drug were retained in the optimized formulation tested with no appreciable changes in frequency. These results suggest that there is no interaction between the drug, polymers and terpenes used in the present study.
Physicochemical properties of transdermal patches
The polymers used for preparation of patches comprised hydrophilic polymers like PVA, PVP, HPMC E15LV and lipophilic polymers like Eudragit RS100, RL100 and hydrophobic polymers like ethyl cellulose. Reproducibility in film properties and sustained release nature was not satisfactory in films prepared with a single polymer hence combinations of polymers were used for the studies as given in Table 1. All formulations given in Table 1 formed patches but LPA1, LPA2, LPA3, LPE1, LPE2 and LPE3 were sticky during removal from the Teflon plate which might be due to water absorption property of PVP K-30 at concentrations used thereby imparting stickiness to patches [17]. Hence these patches were not subjected to further analysis.
LLS transdermal patches exhibited superior crushing strength in comparison to LSH and LLH formulations. Due to a combination of hydrophilic and lipophilic polymers present in LLS patches, these have shown low percentage of moisture uptake and lower water transmission rate which helps the formulations to be stable and prevent them from becoming completely dry.
Transdermal patches containing three different ratios of polymer combinations i.e., 4:1, 3:2, 2:3 were prepared to determine % drug release at the end of 12h. The drug release from patches followed the order: LLH>LSH>LLS. The maximum amount of drug release from LLH formulation in particular LLH3 might be due to increased concentration of hydrophilic polymer (HPMC) and ammonium groups of ERL100 which promote rapid hydration and release. The addition of hydrophilic component to an insoluble but permeable film former tends to enhance the release rates. This is because as the proportion of these polymers in the matrix increased, there was an increase in the amount of water uptake and hydration of the polymeric matrix and thus more amount of drug was released. The prolonged release from LLS patches especially LLS3 is due to the lower proportion of ammonium groups in ERS100 and hence retarded hydration [16]. Hence formulation LLH3 was optimized and further effect of terpenes was observed for LLH3.
Different terpenes like nerolidol, linalool, menthol, farnesol, fenchone, limonene, carvone, geraniol were used in concentration of 2.5% and 5% for the optimized formulation LLH3. From Table 2, the release profile was found to be in the same order as that of boiling point of terpene and hence the hydrogen bonding capacity of terpene. The drug release rate order follws: limonene (hydrocarbon) > fenchone (ketone) > linalool (mono-alcoholic) > menthol (solid mono-alcoholic) > geraniol (mono-alcoholic) > carvone (ketone) > nerolidol (sesquialcoholic) > farnesol (sesqui-alcoholic). Mechanistically terpenes bind with skin lipids leading to the breakage of hydrogen bonds between the ceramide head groups of lipids in the stratum corneum leading to greater fluidization of the lipids, thereby promoting the greater permeation and drug release [18,19]. Hence it can be inferred that higher is the boiling point, higher is the hydrogen bonding capacity and hence the drug release.
| Formulation Code | %Drug content* | Thickness (µm)* | Weight variation (mg)* | Folding endurance* | %Moisture uptake* | WVTR(gm/cm2/h)* | % drug release at 12 h* |
|---|---|---|---|---|---|---|---|
| LLH3N(2.5%) | 99.33 ± 0.22 | 150 ± 0.55 | 238.50 ± 2.01 | >300 | 7.10 ± 0.10 | 0.125 ± 0.04 | 66.48 ± 0.53 |
| LLH3Fa(2.5%) | 99.80 ± 0.22 | 150 ± 0.53 | 238.60 ± 2.18 | >300 | 7.00 ± 0.10 | 0.123 ± 0.09 | 66.01 ± 0.98 |
| LLH3Lm(2.5%) | 99.77 ± 0.20 | 150 ± 0.52 | 238.70 ± 2.10 | >300 | 7.20 ± 0.05 | 0.124 ± 0.05 | 88.50 ± 0.66 |
| LLH3Ln(2.5%) | 99.80 ± 0.22 | 150 ± 0.58 | 238.60 ± 2.13 | >300 | 7.20 ± 0.10 | 0.122 ± 0.05 | 74.11 ± 0.99 |
| LLH3M(2.5%) | 100.86 ± 0.21 | 150 ± 0.57 | 238.80 ± 3.01 | >300 | 7.00 ± 0.05 | 0.120 ± 0.09 | 73.33 ± 0.98 |
| LLH3G(2.5%) | 99.77 ± 0.20 | 150 ± 0.59 | 238.60 ± 3.18 | >300 | 7.26 ± 0.08 | 0.123 ± 0.08 | 69.98 ± 0.18 |
| LLH3C(2.5%) | 101.40 ± 0.21 | 150 ± 0.56 | 238.80 ± 1.33 | >300 | 7.10 ± 0.09 | 0.121 ± 0.02 | 67.25 ± 0.76 |
| LLH3Fe(2.5%) | 98.95 ± 0.20 | 150 ± 0.59 | 238.60 ± 2.15 | >300 | 7.12 ± 0.06 | 0.122 ± 0.06 | 78.44 ± 1.45 |
| LLH3N(5%) | 99.80 ± 0.22 | 150 ± 0.27 | 239.70 ± 2.10 | >300 | 7.23 ± 0.04 | 0.142 ± 0.04 | 70.62 ± 0.98 |
| LLH3Fa(5%) | 99.86 ± 0.21 | 150 ± 0.77 | 239.70 ± 2.12 | >300 | 7.10 ± 0.09 | 0.144 ± 0.09 | 69.93 ± 1.65 |
| LLH3Lm(5%) | 100.17 ± 0.20 | 150 ± 0.62 | 239.80 ± 2.11 | >300 | 7.30 ± 0.05 | 0.147 ± 0.05 | 92.41 ± 0.63 |
| LLH3Ln(5%) | 101.09 ± 0.18 | 150 ± 0.43 | 239.80 ± 2.12 | >300 | 7.34 ± 0.14 | 0.143 ± 0.05 | 77.74 ± 1.67 |
| LLH3M(5%) | 99.33 ± 0.22 | 150 ± 0.66 | 239.60 ± 3.11 | >300 | 7.10 ± 0.09 | 0.148 ± 0.09 | 76.55 ± 1.22 |
| LLH3G(5%) | 98.95 ± 0.20 | 150 ± 0.72 | 239.50 ± 3.08 | >300 | 7.32 ± 0.08 | 0.147 ± 0.08 | 73.67 ± 0.97 |
| LLH3C(5%) | 98.77 ± 0.20 | 150 ± 0.65 | 239.20 ± 1.03 | >300 | 7.20 ± 0.09 | 0.149 ± 0.02 | 72.75 ± 0.97 |
| LLH3Fe(5%) | 99.33 ± 0.22 | 150 ± 0.48 | 239.30 ± 2.05 | >300 | 7.22 ± 0.06 | 0.146 ± 0.06 | 82.48 ± 1.22 |
Table 2: Physicochemical evaluation of transdermal patches with terpenes.
Ex vivo diffusion studies
LLH3Lm(2.5%), LLH3Lm(5%) were taken for the ex vivo studies due to the reason that these formulations has shown cumulative percentage drug release more than 85% within 12 hours. LLH3 was used as the control formulation in order to demonstrate the effect of terpenes on skin permeability of LTG. Limonene is a hydrocarbon lipophilic terpene which enhances the permeation of both lipophilic and amphiphilic compounds [20]. From Figure 2, it can be observed that increased bioavailability and prolonged steady state concentration could be achieved using 2.5%, 5%w/v limonene which is incorporated into a lamotrigine transdermal therapeutic system.
Statistical analysis using one way ANOVA (Tukey’s multiple comparison test): Results from Table 3 suggest that there was a significant difference (P<0.001) in steady state flux, Q12 permeation, enhancement ratio. A significant difference (P<0.01) was observed for permeability coefficient but results were not significant for lag time (P>0.05).
| Parameters | Control Vs LLH3Lm(2.5%) | Control Vs LLH3Lm(5%) | LLH3Lm(2.5%) Vs LLH3Lm(5%) | F value |
|---|---|---|---|---|
| Steady state flux(μg/cm2/h) | *** | *** | *** | 1511 |
| Enhancement ratio (folds) | *** | *** | *** | 571.9 |
| Permeability coefficient | *** | *** | ** | 136.7 |
| Q12 permeation (μg/cm2) | *** | *** | *** | 567.5 |
| Lag time (h) | *** | *** | NS | 99.27 |
Table 3: One way ANOVA by Tukey’s multiple comparison test.
Release kinetics for the optimized formulations calculated from the R2 value suggest that drug release from the system is diffusion limited as it obeys Higuchi model equation. The ‘n’ value of the Korsemeyer- Peppas kinetics indicates that the release follows non fickian diffusion which can be attributed to the combination of lipophilic and hydrophilic polymers used in the formulation.
From Table 4, comparing the results of 2.5% and 5% terpenes for LLH3 formulation in comparison to control, it was observed that as the concentration of the terpene increases the penetration enhancement effect increased [20,21]. These results suggest that, limonene a hydrocarbon lipophilic terpene was found to be ideal to enhance the permeation of lamotrigine.
| Formulation Code | Q12 permeation (μg/cm2)* | SSTF (μg/cm2/h)* | Permeability coefficient (cm/h)* | Lag time (h)* | ER (folds)* |
|---|---|---|---|---|---|
| LLH3(control) | 6274.74 ± 2.06 | 512.43 ± 2.11 | 0.152 ± 0.981 | 3.00 ± 0.98 | 1 |
| LLH3Lm (2.5%) | 8138.20 ± 2.17 | 636.93 ± 2.10 | 0.189 ± 0.762 | 1.20 ± 0.97 | 1.25 |
| LLH3Lm (5%) | 8958.82 ± 2.10 | 725.64 ± 2.22 | 0.215 ± 0.987 | 1.00 ± 0.99 | 1.43 |
Table 4: Ex vivo skin permeation parameters.
Skin irritation studies
Very slight erythema was observed for standard irritant as shown in Figure 3a and no erythema and edema was observed for placebo, control and LLH3Lm(2.5%), LLH3Lm(5%) and it is scored as 0.
Stability studies
It was observed that there was no much difference in the physicochemical properties of patches containing terpenes after stability study. Transdermal patches loaded for stability testing appeared clear and transparent with acceptable folding endurance and % drug content.
Lamotrigine transdermal patches were successfully prepared by solvent casting method using different polymers with terpenes as permeation enhancer. Among polymers used in the study only patches containing combination of lipophilic and hydrophilic polymers LLH, LLS and LSH exhibited satisfactory physicochemical properties. About 67% of drug release was observed for LLH3 formulated without terpenes whereas for LLH3Lm (2.5%) and LLH3Lm (5%) patches containing limonene as enhancer, drug release at the end of 12h was found to be 89% and 93% respectively. This clearly shows the effect of presence of permeation enhancer in the formulation and its concentration in enhancing the drug release. Formulations LLH3Lm (2.5%) and LLH3Lm (5%) when compared with control improved permeability, SSTF and the lag time to drug permeation was reduced to a considerable extent. Based on in vitro and ex vivo permeation studies, Eudragit RL 100 and HPMC E15 LV with limonene at 2.5% or 5% were found to be the optimized formulations. Highest hydrogen bonding capacity of limonene with stratum corneum lipids when compared to other terpenes used in the study might have contributed to enhancement in drug release and skin permeability of drug. A clinically much needed transdermal dosage form of lamotrigine is need of the hour to avoid various pharmacokinetic problems of the drug. Since the optimized formulations were found to be stable in terms of physicochemical properties, further work is recommended to support its efficacy claims by long term preclinical and clinical studies on animals and humans.