
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2023)Volume 14, Issue 2
Introduction: During a craniotomy, the intracranial pressure value is reduced; however, the opening of the compartment may generate transcalvarial herniation with risk of secondary injury by venous compression over the edge of the craniotomy. Cerebral relaxation has been considered an important neuroprotective measure. Historically, cerebral edema has been managed with mannitol; nevertheless, the use of 3% Hypertonic Saline (HS) as first choice therapy is increasing.
Material and methods: Cohort, observational, retrospective, analytical, longitudinal study with a control group. Thirty ASA II-III patient files were included, aged 18 to 60 years, with a diagnosis of supratentorial brain tumor, serum sodium between 135 and 155 mEq/L and clinical intracranial hypertension. The groups were constituted considering the management used and were related by binary logistic regression analysis.
Results: Female gender was the most frequent in both groups; the most prevalent diagnosis in the HS 3% group was meningioma (40%) while in the mannitol group the majority was distributed between meningioma (20%), glioblastoma (20%) and frontal tumors (20%). There was a 1.37-fold advantage of HS 3% over mannitol in improving edema management; however, this advantage was not statistically significant (RR 1.37, 95%CI 0.286-6.6).
Conclusion: Hypertonic saline solutions may be an effective alternative to other conventional osmotic agents, especially in patients with supratentorial brain tumors. Further evidence needs to be generated with controlled clinical studies and an adequate sample size.
Hyperosmolar therapy; Hypertonic saline 3%; Mannitol; Supratentorial parenchymal neoplasia
Tumors of the central nervous system are a worldwide health problem. Each year, about three hundred thousand new cases are diagnosed, corresponding to 2.5% of cancer mortality. In Mexico, according to GLOBOCAN, central nervous system tumors ranked 17th in cancer incidence, with 3,451 (2.01%) cases; during the same year, there were 2,663 (3.46%) deaths, making them the 13th cause of cancer mortality. The average age is 45 years; in our country, the incidence is estimated at 3.5 cases per 100,000 inhabitants and represents the second and fifth cause of cancer mortality in the 0-18 and 18-29 age groups, respectively. Brain tumors are divided into malignant and nonmalignant. The most common worldwide are meningiomas, most of them are non-malignant. In second place come those of the pituitary gland, and in third place are glioblastomas. In this type of pathology, there is a high incidence of increased intracranial pressure due to failure of compensatory mechanisms and the underlying pathology itself.
Cerebral Blood Flow (CBF) and cerebral blood volume are of great importance in patients with elevated Intracranial Pressure (ICP) because of the risk of developing cerebral edema or increasing it during surgery, thus increasing the risk of causing perioperative cerebral ischemia [1]. One of the important goals of anesthetic management in patients undergoing craniotomy is to provide a relaxed brain in which the surgeon has an adequate surgical field. This allows easy surgical manipulation and causes less damage to healthy brain tissue, thus resulting in less secondary injury to the brain, which improves the patient's neurological outcome. The administration of osmotherapy at the beginning of craniotomy is one of the interventions used to produce brain relaxation, which prevents the surgeon from working with a tense brain [2]. Mannitol has generally been considered a "gold standard" for decreasing brain tissue tension and endocranial hypertension; however, hypertonic saline is another intravenous fluid that has comparable effects to mannitol in terms of reducing intracranial pressure [3].
Osmolarity is the main determinant of water movement across the intact blood-brain barrier and is predictable. If serum osmolarity is transiently increased, normal brain tissue would become dehydrated, reducing brain volume and thereby reducing ICP [4].
Osmotic solutions can be classified by their tonicity, the "effective osmotic forces exerted by solutions in contiguous compartments". Tonicity is expressed by the "osmotic reflection coefficient" (σ) with values ranging from 0 (a freely permeable particle with no osmotic force) to 1 (a completely impermeable particle with ideal osmotic activity). Mannitol has a reflection coefficient of 0.9 and sodium chloride has a reflection coefficient of 1 and the latter is theoretically an ideal osmotic agent and more effective than mannitol [5-11].
Administration of 3% hypertonic saline or mannitol increases serum concentration or osmolarity and decreases ICP as well as water content in healthy brain parenchyma. The principal mechanism underlying these effects is the induction of a shift of water from brain tissues into the intravascular space by the hyperosmolarity of hypertonic solutions due to the blood-brain barrier being impermeable to sodium and mannitol. The increase in serum sodium following the use of 3% hypertonic saline stimulates the release of antidiuretic hormones, which leads to the absorption of free water from the kidney, which may explain a lesser diuretic effect [5]. Given these premises, mannitol has become the traditional basis for hyperosmolar therapy. However, it may be associated with serious adverse effects such as decreased intravascular volume, paradoxical elevation of intracranial pressure and renal failure [3]. On the other hand, Hypertonic Saline (HS) has been used more frequently to lower ICP in patients with traumatic brain injury or transoperative cerebral edema and/or as adjunctive therapy to mannitol use, either sequentially or in combination. Bolus dosing has been used in different concentrations, with none showing superiority over another, and the total osmolar load should be considered [6].
Measurement of serum osmolarity is recommended in patients with brain injury, renal failure, hepatic failure or shock states because serum osmolarity may be unexpectedly elevated [9].
The perfusion of 3% HS has been effective at 1-3 ml/kg bolus, titrating the escalating doses to a value between 145-155 mEq/L of Sodium (Na) maximum 160 meq/L and at an osmolarity of 320-330 mOsm/L (maximum 360 mOsm/L) [6]. In theory, HS acts similarly to mannitol, producing outflow of water from the nervous tissue to the intravascular space and reducing the rate of Cerebrospinal Fluid (CSF) production, thus improving intracranial compliance. It also has a lower diuretic effect, so it initially has the advantage of expanding the intravascular volume and increasing mean arterial pressure, Cardiac Output (CO) and CBF while it decreases ICP. Nevertheless, there is no conclusive evidence that demonstrates the superiority of management with HS over mannitol, so the objective of this study was to identify whether there are differences in the control of cerebral edema between treatment with HS 3% and mannitol in patients with Supratentorial parenchymal neoplasms [6,8].
This is a cohort, observational, analytical, retrospective and longitudinal study. The sample size was calculated with the formula for comparison of proportions [7], with a 95% confidence level, 80% power, with a two-tailed hypothesis, an expected prevalence of satisfactory relaxation (p1) of 90% for HS and one (p2) of 80% (for mannitol) [8], which gave us a sample of 19 subjects per group.
The data were obtained from the records corresponding to patients operated on by the neurosurgery service during the period March 2019 to March 2020 at the Hospital General Balbuena in Mexico City because the intervention of patients was interrupted, due to issues arising from the SARS-CoV-2 pandemic disease COVID-19, it was only possible to collect information from 30 patients (15 cases and 15 controls) which are analyzed and presented below, as an interim analysis.
Records of ASA II-III patients between 18 and 60 years old, with a diagnosis of supratentorial parenchymal neoplasm, with serum sodium between 135 and 155 mEq/L, clinically with intracranial hypertension data and with no evidence of having received hyperosmolar fluid perfusion 24 hours before the surgical event were included. We excluded patients with cardiac or renal failure, shock, as well as with a Glasgow Coma Scale (GCS) on admission less than 12 points. All procedures were performed in a standardized manner, by the same team of anesthesiologists.
No premedication was used in any case. In the operating room, standard monitoring was employed, consisting of PANI, 2-lead EKG (DII and V5), pulse oximetry, plethysmography, capnography, capnometry, diuresis, Train of 4, as well as measurement of water-electrolyte/acid-base balance by arterial gas sampling. All patients were admitted with central venous catheter for administration of hyperosmolar solutions. Anesthesia was induced with fentanyl 3 mcg/kg, lidocaine 1 mg/kg, propofol 1 mg/kg and rocuronium 0.7 mg/kg body weight. Anesthesia was maintained with sevofluorane 1.0 MAC with oxygen mixture, and subsequent doses of rocuronium were used according to the train-of-four ratio. Patients were mechanically ventilated to maintain a partial pressure of carbon dioxide between 30 and 35 mmHg.
The control group received mannitol 0.5 g/kg 15 minutes before opening of the dura and the case group received hypertonic saline 3% 2 ml/kg, 40 minutes before opening of the dura, through a central line as a bolus. The response to treatment was evaluated by the neurosurgeon through a scale, where the following parameters were considered:
1) Cerebral edema; from observation, the characteristics of cerebral expansion and relationship with the internal table of the bony rim, 2) hourly uresis, 3) serum sodium measurement and 4) serum osmolarity. With the mentioned indicators a score is determined which results in a response: good (12-10 points), regular (9-7 points) or bad (less than 7 points). To determine the association between variables, the result was dichotomized and analyzed using binary logistic regression (RR 95% CI).
Thirty files corresponding to 15 patients treated with HS 3% and 15 patients treated with mannitol were analyzed, the female gender was the most frequent in both groups (66.7% and 60% respectively); most of the patients were classified as ASA II, with no statistical differences between groups (Table 1). The most frequent diagnosis in the HS 3% group was meningioma (40%) while in the mannitol group the majority was distributed between meningioma (20%) glioblastoma (20%) and frontal tumors (20%).
| N= 30 | SSH 3%, n=15 | Mannitol, n=15 | p | |
|---|---|---|---|---|
| Sex | Male | 33.30% | 40.00% | 0.5 |
| Female | 66.70% | 60.00% | 00 | |
| ASA | ||||
| II | 46.70% | 40.00% | 0.5 | |
| III | 53.30% | 60.00% | 00 | |
| Diagnostic | ||||
| Astrocytoma | 6.70% | 6.70% | ||
| Glioblastoma | 20.00% | 20.00% | ||
| Craniopharyngioma | 6.70% | - | ||
| Meningioma | 46.70% | 20.00% | ||
| Oligodendroglioma | - | 6.70% | ||
| Tumor temporoparietal | 6.70% | - | ||
| Frontal tumor | 6.70% | 20.00% | ||
| Parietal tumor | 6.70% | 13.30% | ||
| Temporary tumor | 6.70% | 6.70% |
Note: * The difference between percentages was calculated with Chi-square.
Table 1: General characteristics of the study population.
Among the pre-surgical clinical conditions, baseline GCS values, baseline sodium, glucose and osmolarity were recorded and used to evaluate the follow-up. No statistically significant pre-surgical differences were observed between the study groups (Table 2). On the other hand, we can observe that no pre-surgical differences were found between the mannitol group and the HS group; the differences between groups were statistically significant only in the post-surgical measurements of sodium (p=0.00), glucose (p=0.014) and osmolarity (p=0.001).
| N= 30 | HS 3%, n=15 | Mannitol, n=15 | p |
|---|---|---|---|
| Glasgow Coma Scale | 14 ± 1.2 | 13 ± 1 | 0.28 |
| Pre-surgical sodium (mEq/L) | 137 ± 3 | 139 ± 2 | 0.241 |
| Initial glucose (mg/dl) | 114 ± 23 | 134 ± 17 | 0.101 |
| Pre-surgical Osmolarity (mOsm/L) | - | - | - |
| Post-surgical sodium (mEq/L) | 281 ± 6 | 285 ± 6 | 0.147 |
| Post-surgical glucose (mg/dl) | 145 ± 2 | 141 ± 2 | 0 |
| Osmolarity post-surgery (mOsm/L) | 130 ± 13 | 143 ± 14 | 0.014 |
Note: *Comparison between means was performed with Student's t-test for independent samples.
Table 2: Pre-surgical conditions of patients diagnosed with supratentorial parenchymatic neoplasia differences according to management.
The pre, trans and postoperative sodium values were compared by study group, showing a statistically significant difference between the postoperative and the preoperative values. Sodium elevation was more evident in the group treated with HS 3% (from 139 meq/L to 141 meq/L in the mannitol group (p=0.213) and from 137 meq/L to 145 meq/L in the HS group, p=0.04) (Figure 1). Similarly, a pre- and post-surgical difference was observed in the glucose value (p=0.012), especially in the mannitol group (p=0.03) (Figure 2). Regarding osmolarity, an increase was observed in both groups at the different stages. However, although initially the osmolarity of the group treated with HS 3% was lower compared to the group treated with mannitol, the post-surgical values reached were higher, with statistically significant differences between groups (p=0.001) (Figure 3). There were no differences in the final uresis values between both groups (Figure 4).
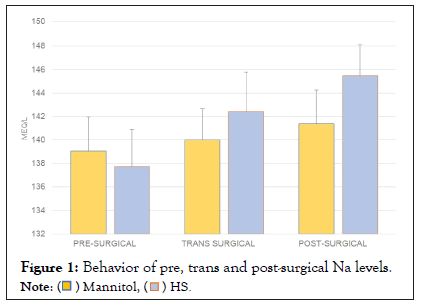
Figure 1: Behavior of pre, trans and post-surgical Na levels.
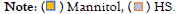
Figure 2: Behavior of pre, trans and post-surgical glucose levels.
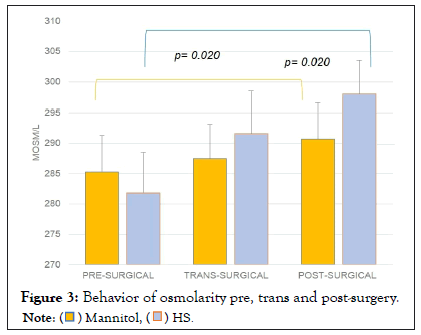
Figure 3: Behavior of osmolarity pre, trans and post-surgery.
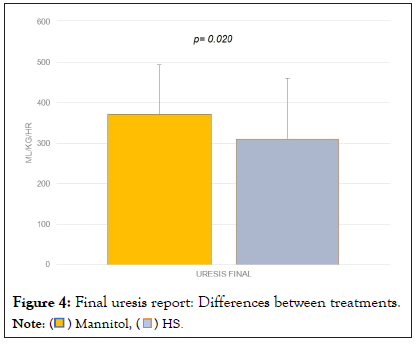
Figure 4: Final uresis report: Differences between treatments.
Finally, the dichotomization between the greater or lesser tension of the encephalic mass was performed to compare whether HS 3% represented an advantage against mannitol. Tension assessment was 1.37 times better for HS 3% than mannitol, but this advantage was not statistically significant (RR 1.37, 95%CI 0.286-6.6) (Table 3).
| N=30 | Mannitol | HS 3% | p | RR (IC95%) |
|---|---|---|---|---|
| Good edema control | 66.70% | 73.30% | 0.69 | 1.37 (0.286 - 6.6) |
| Poor edema control | 33.30% | 26.70% |
Table 3: Estimated advantages between manitol and hs 3% in the study population.
The term cerebral edema implies an increase in water content in the brain, leading to expansion of brain volume. Cerebral edema can occur focally or diffusely, secondary to a primary brain injury, or in some systemic diseases, either acute or chronic. Identification of cerebral edema is important because it is a major cause of secondary brain injury. It can cause compression of brain structures, anatomical modifications, herniation of brain tissue and compromise of cerebral blood flow through increased ICP [9]. In the case of our patients, there was a good prevention of edema and no symptoms related to compression of structures or compromised flow was detected.
According to Liotta, there are four forms of cerebral edema: vasogenic, cytotoxic, hydrostatic and osmotic [9]. Vasogenic and cytotoxic edema are the most common, identifying the dominant type of brain edema based on the neuroimaging pattern and mechanism of injury can guide initial treatment strategies to minimize secondary brain injury. Our patients had the common denominator of the primary tumor, so we consider that the treatment scheme should be directed to prevent vasogenic edema.
The Monro-Kellie theory affirms that the cranial cavity is rigid, and the intracranial components can be modifiable up to a certain limit, these components are: cerebral parenchyma, cerebral blood flow and cerebrospinal fluid; which give us cerebral compliance [6]. Vasogenic edema responds to steroids (especially tumor edema) as well as to osmotherapy, so it is adequate and biologically plausible to think favorable results with treatment based on HS 3% or mannitol.
Prior considerations for the use of osmotic agents should include desirable characteristics, which include being pharmacologically inert, non-toxic and having a short elimination time [10]. Less solute leakage may result in a greater increase in serum osmolarity, and a greater transendothelial osmotic gradient in the vascular compartment may lead to greater extraction of brain water into the intravascular space. In this regard, our data revealed a more effective brain volume reduction associated with hypertonic saline 3% versus mannitol, which is consistent with the classical theory of hyperosmolar therapy [8].
Several authors have generated evidence of the use of hypertonic saline with different concentrations of 3%, 7.5% or 23.4% and each of them represents a different osmotic activity. Regardless of the type of osmotically active agent, the main objective of osmotherapy is to maintain plasma osmolarity within the range of 300 to 320 mOsm/kg [11]. We can observe that during the trans and postoperative period, the group treated with HS 3% was maintained with an osmolarity between 290 and 302 mOsm/kg, which is highly recommended. It is important to emphasize that in all patients with brain injury, measurement of serum osmolarity is recommended, especially if they are at risk of renal failure, hepatic failure or shock states because serum osmolarity may be unexpectedly elevated [9].
Ziai, et al. mention that therapeutic concerns with mannitol include major systemic side effects such as hypotension, hemolysis, hyperkalemia, renal failure and pulmonary edema; while side effects of hyperosmolar saline therapy can range from neurological, cardiac and immunological conditions [6]. One of the important complications with the use of HS, is the presentation of pontine myelinolysis; osmotic demyelination syndrome due to rapid overcorrection of pre-existing hyponatremia [8]. Other possible neurological complications of rapid changes in sodium and plasma osmolarity include symptoms and signs of encephalopathy (confusion, lethargy, seizures, and sometimes coma). Figure 3 shows that the osmolarity of the group treated with HS 3% started at around 282 mOsm/l and that the most significant difference reached was 10 mOsm/l compared to the next measurement (trans-surgical), and then reached 298 mOsm/l, well below the figure considered to be at risk.
Likewise, no side effects associated with osmolar overcorrection were observed, neither with mannitol nor with HS 3%. Studies have found that the administration of HS vs. mannitol is safer [6]. In the case of our patients, no undesirable side effects were observed.
In a Cochrane review by Chen et al., which aims to compare hypertonic saline against other intracranial pressure lowering agents, specifically mannitol, the authors conclude that in the face of acute brain injury there is strong evidence that hypertonic saline is better than mannitol in terms of efficacy and safety [10]. Unfortunately, our results cannot affirm such superiority, although they can suggest it by obtaining in the contingency analysis a RR of 1.37 (0.286-6.6), this width of the confidence interval may be due to a small sample size, which as mentioned at the beginning of the article had to stop performing scheduled surgery because of the pandemic, so it is suggested that it is not that the working hypothesis was not fully tested, but it is considered likely to be committing a type II error (associated with a small sample size).
The limitation of the clinical studies reviewed precludes us from making a recommendation of preference of one agent over another. A large randomized controlled study is needed to make recommendations. While we await such clinical evidence, there are numerous biochemical, physiologic, and side effect considerations that physicians should be aware of when selecting the most appropriate hyperosmolar therapy for their patients.
Finally, we would like to emphasize that it has been described that an increase in plasma osmolarity can affect cardiac, renal, immune function; nevertheless, a deleterious effect of mannitol-induced hyperosmolality has only been clinically documented regarding renal and cardiac function. An increase in plasma osmolarity after mannitol administration above 313 mOsm/kg significantly increases the risk of prolongation of the corrected QT interval above 500 ms, which is associated with the incidence of atrial fibrillation in patients without a cardiac antecedent. At serum osmolarity above 320 mOsm/L, hyperosmotic stress has been documented to be associated with hyperosmotic stress is associated with the secretion of proinflammatory cytokines, such as: TNF, IL1-β, IL-6 and IL-8 [11,12].
Although the importance of the management of cerebral edema and the potential damage resulting from it has already been mentioned, we must contemplate that the surgical prognosis in a brain tumor resection will always be influenced by several factors, including the size and location of the tumor, the tumor lineage, the severity of adjacent tissue damage, and the immune and inflammatory response.
Hypertonic saline solutions may be an effective alternative to other conventional osmotic agents, especially in patients with supratentorial brain tumors or transoperative cerebral edema. Controversies about the use of hypertonic saline solutions as first-line agents exist largely because of the paucity of clinical studies comparing it with conventional therapies. The results obtained in this study, may suggest more, do not generate sufficient evidence to give a solid recommendation for first-line use of HS 3%; we recommend for future research a larger sample size and timely follow-up of serum osmolarity and electrolyte values, with each of the interventions performed.
The authors declare that there is no conflict of interest. They also declare that no funding was received for the development or completion of this study.
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
Citation: del Socorro GCM, Rosalinda JA, Antonio CSJ, Libertad TSQ, Uriel HGA, Ricardo RA, et al. (2023) Transoperative Management of Cerebral Edema Secondary to Supratentorial Tumors Hypertonic Saline 3% vs. Mannitol: A Reconstruction of a Cohort. J Anesth Clin Res. 14:1098.
Received: 13-Feb-2023, Manuscript No. JACR-23-21778; Editor assigned: 15-Feb-2023, Pre QC No. JACR-23-21778 (PQ); Reviewed: 22-Feb-2023, QC No. JACR-23-21778; Revised: 08-Mar-2023, Manuscript No. JACR-23-21778 (R); Published: 15-Mar-2023 , DOI: 10.35248/2155-6148.23.14.1098
Copyright: © 2023 del Socorro GCM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.