Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2020)Volume 5, Issue 2
This study was conducted to evaluate implementation of the bronchiolitis pathway for the management of infants with bronchiolitis. Seventy-eight patients were stratified into two cohorts: group A, before implementation of the pathway, and group B, after implementation. The Modified Pulmonary Index Score (MPIS) was used to assess patients’ level of respiratory distress and response to therapy. The median length of stay did not differ significantly between the two groups (p=0.6). However, there was a statistically significant difference in the transfer rate of patients to the Pediatric Intensive Care Unit (PICU) with 3% in group A compared to 20% in group B (p = 0.03). Although implementation of our protocol did not result in decrease in LOS or medication use, we believe that the pathway may have heightened clinicians’ perception for the severity of bronchiolitis and likely resulted in increased PICU admission. In the future, additional modifications to our protocol will be made based on updated AAP recommendations. This study has laid the foundation for continued monitoring and tailoring of a pathway to address the needs of pediatric patients with bronchiolitis at other institutions.
Bronchiolitis; Modified pulmonary index score; Pediatrics; Respiratory syncytial virus; Pediatric Intensive Care Unit; PICU
American Academy of Pediatrics (AAP); Institutional Review board (IRB); Length of Stay (LOS); Modified Pulmonary Index Score (MPIS); Pediatric Intensive Care Unit (PICU); Respiratory Syncytial Virus (RSV); Spartanburg Regional Healthcare System (SRHS).
Bronchiolitis is a common viral lower respiratory tract infection seen in infants. This illness typically affects the small airways of the lungs and causes acute inflammation and necrosis of the cells that line them [1]. Bronchiolitis can also cause increased mucus production, which can progress into tachypnea, wheezing, rales, and use of accessory muscles, and/or nasal flaring, indicating that the child is in respiratory distress [1].
Bronchiolitis is the most common cause of hospitalizations among infants during the first year of life [2]. It can be caused by a variety of respiratory viruses, the most common being respiratory syncytial virus (RSV). RSV is more common during the winter months with infectivity lasting into early spring [1]. Approximately 20% to 30% of children diagnosed with RSV experience a lower respiratory tract infection such as bronchiolitis.1 Some of the other more common viruses that can cause bronchiolitis include human rhinovirus, human metapneumovirus, influenza, adenovirus, coronavirus, and parainfluenza viruses [3].
In October 2006, the American Academy of Pediatrics (AAP) published a treatment guideline related to the care of infants with bronchiolitis [3]. This publication was an extensive review of literature available related to this disease. It was endorsed by the Academy of Family Physicians, the American College of Chest Physicians and the American Thoracic Society. The guideline heavily emphasized frequent assessment of infants and children hospitalized for bronchiolitis. It also recommended assessment before intervention as well as an assessment after an intervention. In November 2014, the AAP published a revised and updated Clinical Practice Guideline providing an evidence-based approach to the diagnosis, management, and prevention of bronchiolitis in children from 1 month through 23 months of age [4].
Both guidelines emphasized the importance of using clinical judgment based on history and physical exam to diagnose and assess the patient’s bronchiolitis disease severity. In addition, both guidelines discouraged the use of unnecessary medications and interventions such as steroids, antibiotics, bronchodilators, chest physiotherapy and continuous pulse oximetry monitoring if supplemental oxygen is not required. While these guidelines were very similar, there were a few key changes worth addressing.
The most noteworthy change made in 2014 was regarding the use of beta- adrenergic agonist medications in children. In 2006, these medications were considered optional if patients were carefully monitored and use was continued when there was a documented clinical response using an objective means of evaluation. In 2014, this recommendation was updated to state that clinicians should not administer albuterol, salbutamol, or epinephrine in infants and children with a diagnosis of bronchiolitis. Another important update in 2014 included a statement addressing the use of nebulized hypertonic saline in these patients. Although stated as a weak recommendation, any mention of this modality was absent in the 2006 guideline.
After a review of the various medical treatments for this disease practiced at our institution, Spartanburg Regional Healthcare System (SRHS), a pathway was developed for this institution’s practice primarily based on the 2006 AAP recommendations. This study was conducted to evaluate the difference in length of stay and treatment modalities before and after the SRHS bronchiolitis pathway was executed.
Approval to perform this study was obtained from the institutional review board (IRB) at SRHS. Due to the retrospective nature of the project, a waiver of the requirement to obtain written informed consent was granted by the IRB. Appropriate training was performed by all investigative parties before data collection began.
The study was a single-center, retrospective, observational study, conducted at a 540-bed community-based teaching hospital. Two seasons were included: before implementation of the pathway, January 2014 - March 2014 (group A), and after implementation of the pathway, January 2015 - March 2015 (group B). Inclusion criteria included: infants less than one year of age admitted to the pediatric ward with the primary diagnosis of bronchiolitis. Exclusion criteria included: age greater than one year, known asthma, heart disease, airway abnormalities, immune deficiency, severe lung disease (Cystic Fibrosis, Chronic Lung Disease), or any chronic comorbidities. Data were de-identified after chart review was complete and then stored in a password protected Excel document, with access given only to approved investigative parties. A total of 78 cases were reviewed. After collection, data was analyzed by a research statistician.
Main study outcomes and comparisons included length of hospital stay, transfer rate to the Pediatric Intensive Care Unit (PICU), and treatment utilization. Modified Pulmonary Index Score (MPIS) was used to assess a patient’s level of respiratory distress and their response to therapy in the second season of our study (group B) (Figure 1). Actual values were inconsistently documented by clinicians; thus, values were not included in this analysis. The pathway developed by SRHS was used throughout this study and assisted in determining patient treatment and appropriate escalation based on the MPIS scores for group B (Figure 2).
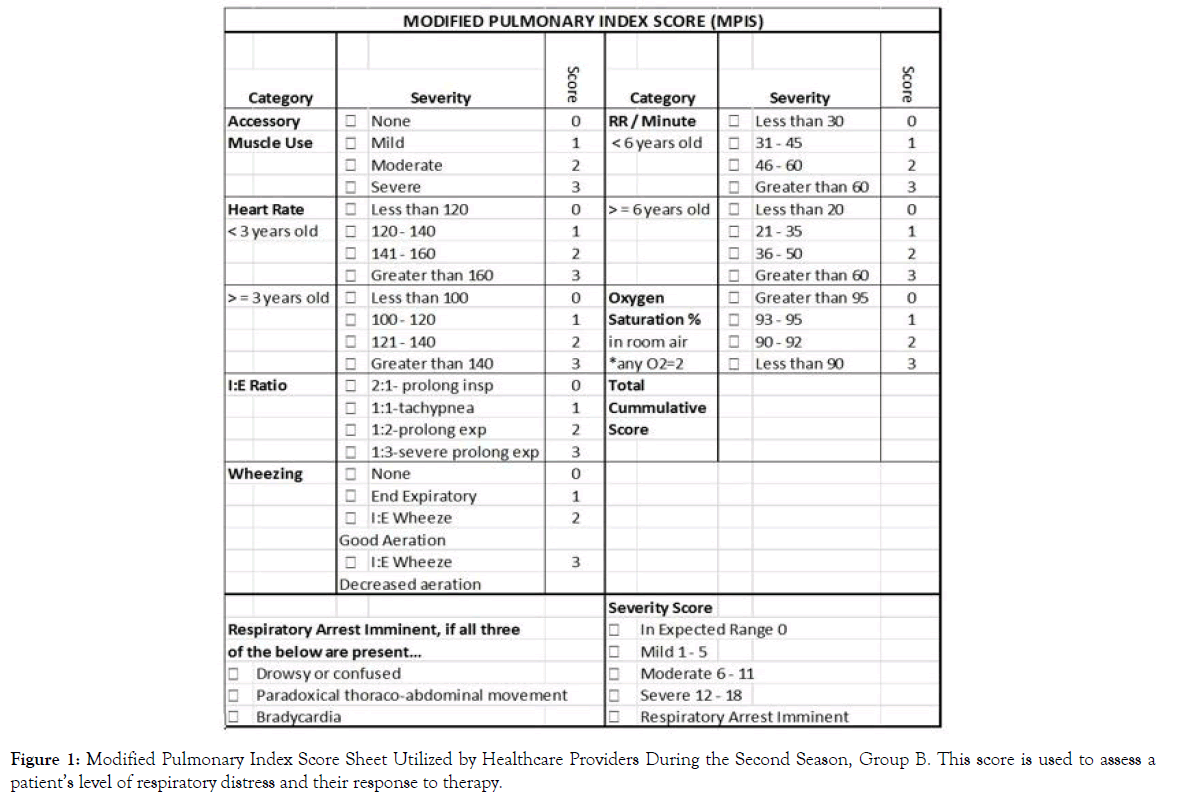
Figure 1. Modified Pulmonary Index Score Sheet Utilized by Healthcare Providers During the Second Season, Group B. This score is used to assess a patient’s level of respiratory distress and their response to therapy.
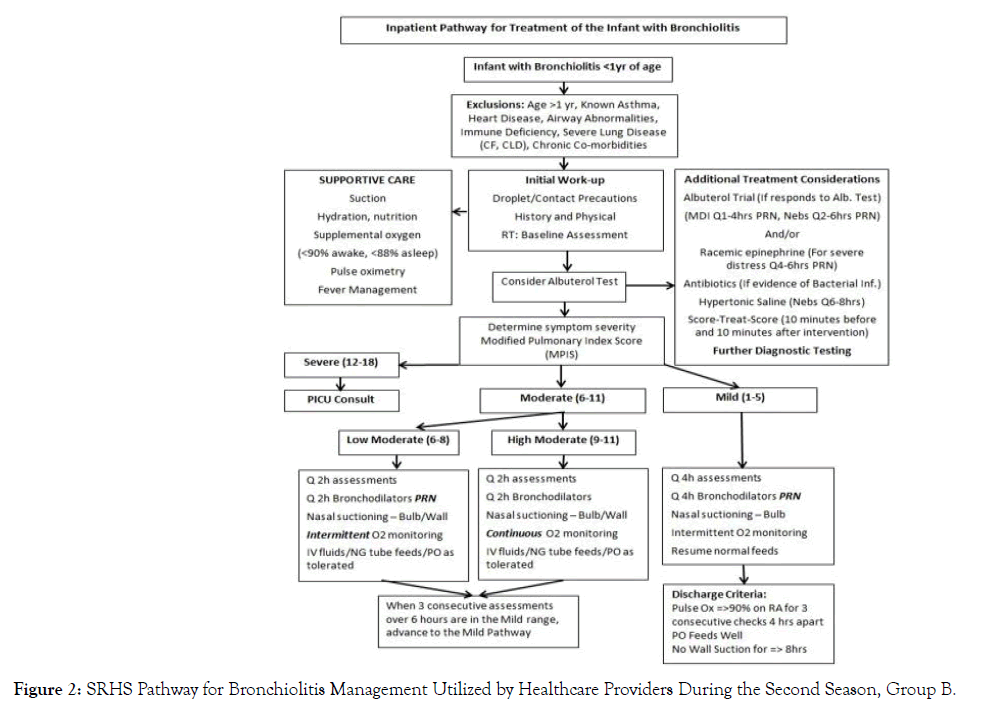
Figure 2. SRHS Pathway for Bronchiolitis Management Utilized by Healthcare Providers During the Second Season, Group B.
A total of 78 patients were included in this retrospective study. Group A included the children treated for bronchiolitis before implementation of the SRHS bronchiolitis pathway and group B included the children treated for bronchiolitis after its implementation. Group A consisted of 43% males and 56% females, of which 62% were Caucasian, 16% were African American, 14% were Hispanic, 3% were Asian, and 5% were listed as other. Group B consisted of 51% males and 49% females, of which 63% were Caucasian, 24% were African American, no patients were Hispanic, 5% were Asian, and 7% were listed as other. All patients included in this retrospective study were under one year of age with 86% of patients that were born full-term (37 - 42 weeks) in group A and 83% of patients were born full-term in group B. Only 14% and 17% of patients were born pre-maturely in groups A and B, respectively. Group A had 92% of patients that tested positive for RSV and 8% that tested negative. All 41 patients in group B were positive for RSV (Table 1). None of the above categorizations resulted in a statistically significant difference between those in groups A and B (p > 0.05).
| Group A | Group B | Total | |
| Totals | 37 | 41 | 78 |
| Gender | |||
| Male | 16 | 21 | 37 |
| Female | 21 | 20 | 41 |
| Race | |||
| Caucasian | 23 | 26 | 49 |
| African American | 6 | 10 | 16 |
| Hispanic | 5 | 0 | 5 |
| Asian | 1 | 2 | 3 |
| Other | 2 | 3 | 5 |
| Gestational Age | |||
| Full Term | 32 | 34 | 66 |
| Premature | 5 | 7 | 12 |
| RSV Status | |||
| Positive | 34 | 41 | 75 |
| Negative | 3 | 0 | 3 |
Table 1: Demographics for Patients with Bronchiolitis Admitted to the General Pediatric Ward or Pediatric Intensive Care Unit Before Implementation of the Pathway, January 2014 - March 2014 (group A), and After Implementation of the Pathway, January 2015 - March 2015 (group B).
The median length of stay before and after implementation of the treatment pathway did not differ significantly; median of 2.0 for both groups A and B (p= 0.6) (Figure 3). The transfer rate to the PICU varied significantly between the two cohorts; 3% of those in group A were admitted to the PICU compared to 20 % of patients in group B (p = 0.03). There were no statistically significant differences between the two cohorts in terms of treatment utilization (p > 0.05) (Figure 4).
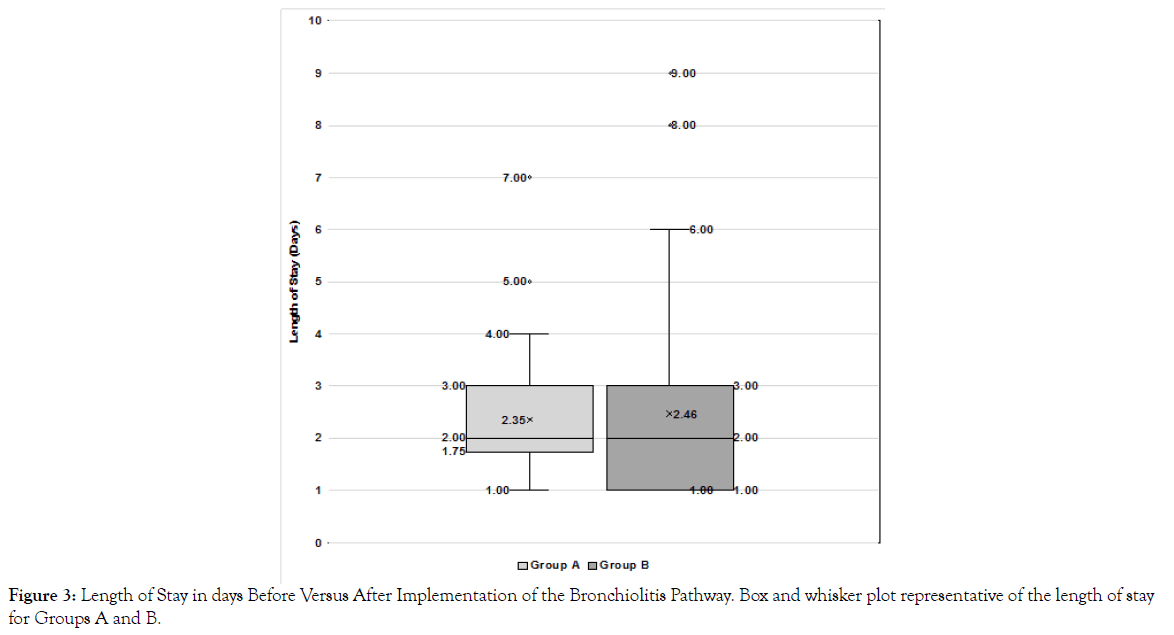
Figure 3. Length of Stay in days Before Versus After Implementation of the Bronchiolitis Pathway. Box and whisker plot representative of the length of stay for Groups A and B.
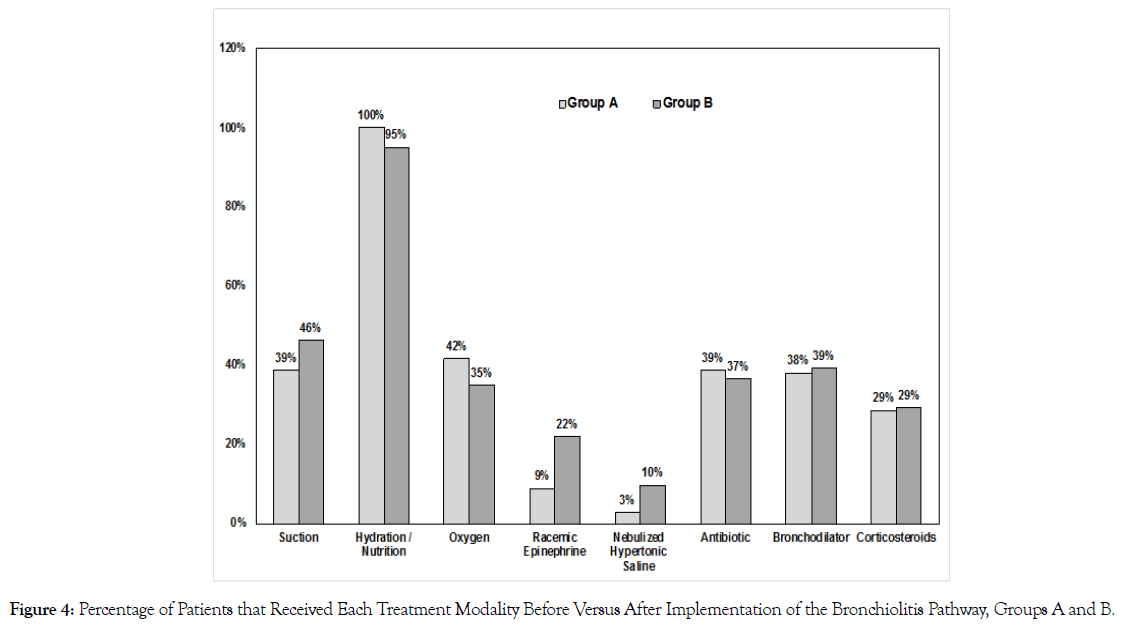
Figure 4. Percentage of Patients that Received Each Treatment Modality Before Versus After Implementation of the Bronchiolitis Pathway, Groups A and B.
Our institution’s bronchiolitis pathway was developed primarily based on the recommendations found in the American Academy of Pediatrics (AAP) 2006 Clinical Practice Guideline regarding the diagnosis, management and prevention of bronchiolitis [3]. One of the benefits of this study and the development of the SRHS pathway is the consideration of a patient’s severity of illness with quantitative measures by using the Modified Pulmonary Index Score (MPIS), and subsequently placing infants into corresponding care groups for treatment. Utilizing this scoring system was initially introduced at our institution due to the 2006 guideline that emphasized the importance of frequent assessment of patients, including assessments both before and after each documented clinical intervention. In 2005 Carroll et al. developed the MPIS to assess the severity of acute asthma exacerbations in children [5]. Our institution values the consistency, reproducibility and objective nature of this scoring system. We also incorporated it into our pathway due to the potential connection between asthma and bronchiolitis. Researchers have investigated the correlation between bronchiolitis in infancy and the risk of developing recurrent wheezing and asthma. A meta-analysis published by Florin et al. determined that 17% - 60% of children hospitalized with bronchiolitis may develop recurrent wheezing later in childhood [6]. Although a correlation has not been definitively proven, utilizing the MPIS values provides us with an objective and consistent means to evaluate children with bronchiolitis.
The MPIS system was implemented during our study’s second season in which group B was analyzed. At our institution, an MPIS greater than 11 allows clinicians to have an objective means to validate transfer to the PICU. In this study, there was a statistically significant increase in the percentage of patients transferred to the PICU for a heightened level of care in group B. The use of this scoring system may have contributed to the increase in the number of patients transferred to the PICU in the second season of our study. It is important for patients with an MPIS greater than 11, categorized as severe, to receive a higher level of care. Unfortunately, this study was conducted before an updated electronic medical record was put into place, thus the MPIS scores were not documented consistently as they are today. Additional studies should be performed at our institution to determine if these outcomes remain true with the exact MPIS values included in the analysis.
Our hospital’s pathway was developed prior to the release of the 2014 AAP bronchiolitis management recommendations [4]. However, when analyzing the results of this study it is important to consider the most updated guideline published by the AAP. Both the 2006 and 2014 guidelines emphasized against using unnecessary medications and interventions when treating infants and children hospitalized for bronchiolitis. Our institution’s pathway was also implemented in order to decrease the unnecessary use of interventions and rely heavily on supportive care. The 2014 AAP Clinical Practice Guideline stated that the initial use of aggressive medical management is not necessary when treating infants with bronchiolitis because clinical benefits have not been consistently documented.
The differences between these two guidelines should also be addressed. As previously mentioned, in 2014 the AAP included the addition of nebulized hypertonic saline and the appropriate application of its use in the inpatient setting, as well as the dissuasion of the use of beta- adrenergic agonist medications. The proposed mechanism of hypertonic saline includes its ability to reduce sputum viscosity, restore the airway’s surface liquid, increase the mucociliary clearance, and trigger the cough reflex.6 Support for this change includes a systematic review and meta-analysis by Zhang et al. that showed a statistically significant decrease in average LOS among infants treated with hypertonic saline, compared to those treated with normal saline or standard care [7]. The SRHS pathway also includes a consideration for nebulized hypertonic saline for all admitted patients with bronchiolitis. Additionally, evidence supporting the change regarding the use beta- adrenergic agonist medications included in the 2014 Cochrane Collaboration systematic review [8]. This review did not identify any evidence in favor of using bronchodilators, primarily salbutamol and excluding epinephrine, for infants admitted to the hospital or in the outpatient setting. The SRHS pathway includes the consideration of racemic epinephrine for bronchiolitis patients in severe distress. The pathway also states the option of an albuterol trial in all of these patients and allows the healthcare provider to escalate its use at their discretion based on the patient’s MPIS value. We realize the SRHS pathway includes these treatment modalities that the AAP recommended against using. When analyzing our institution's use of medications before and after implementation of the SRHS pathway as well as the patient’s length of stay, the results did not show a statistically significant difference. From this it may be inferred that the healthcare providers at our institution have always been relying on the use of supportive care and thus minimizing the overuse of unnecessary interventions even before the release of the updated AAP guideline. Overall, it is important to remember that the AAP Clinical Practice Guideline is only a recommendation for the management of children with bronchiolitis and is used to help clinicians in decision making. However, it does not replace clinical judgment when caring for infants and children with bronchiolitis, especially in severe cases.
The importance of this study also lies in the standardization and streamlined treatment of patients diagnosed with bronchiolitis. Interestingly, our results did not find statistically significant differences in terms of medication administration or length of stay between groups A and B. Multiple factors may have played a role in our results, such as the inability to capture the precise MPIS values for patients in our study and our small sample size. Additionally, we believe that our pathway may have heightened clinicians’ perception for the severity of bronchiolitis and likely resulted in increased PICU admission. We believe our study has laid the foundation for continued monitoring and tailoring of this pathway to address the needs of patients with bronchiolitis at other institutions. Further studies should expand on our model to utilize a similar process and explore the benefits of giving their healthcare providers a pathway that eliminates subjectivity and allows MPIS assessments to guide the management of patients with bronchiolitis and further escalate treatment when necessary. In the future, additional modifications to bronchiolitis protocols should be made, as applicable, based on any updates published by the AAP.
No external funding received.
We would like to thank the original data collection researchers (Danielle Tamburrini, DO, Heather Vannoy, DO, Kiesha Gray- Anderson, DO, and Kim Cantrell, RRT-NPS) and VCOM research statisticians for their help in analyzing the data. We would also like to thank the PICU and Pediatric Nurses at Spartanburg Medical Center for all their hard work in caring for the infants and children.
Citation: Quigley M, Driscoll HC, Sahhar HS (2020) Treatment and Medical Management of Infants with Bronchiolitis: Effect of Pathway Implementation on Length of Hospital Stay and Transfer Rate to Critical Care. Clin Pedia OA 5:165 doi: 10.35248/2572-0775.20.5.165
Received: 10-Apr-2020 Accepted: 23-May-2020 Published: 30-May-2020 , DOI: 10.35248/2572-0775.20.5.165
Copyright: © 2020 Quigley M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Competing interests: The authors have declared that no competing interests exist.