Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2023)Volume 14, Issue 5
Background: Striae distensae is a common dermatological impairment leading to a therapeutic challenge. Many treatments are available; however, these treatments usually incur risks of hyperpigmentation and bad scar healing related to the phototype of the patient. Poly-L-Lactic Acid (PLLA) could improve the area due to its biostimulatory properties.
Objective: To demonstrate the improvement of white striae after PLLA application.
Method: This was a pilot study conducted in adults with symmetrical and bilateral white striae, 1 mm-4 mm wide, located on the gluteal or abdominal region. Photographs for register of the improve, a questionnaire about adverse events, and ultrasonography, to analyze the skin thickness of the striae area pre and post application, were done before and after 3 and 6 months of the last application of PLLA. Three monthly applications were performed in the affected area, using 0.04 ml of the product retro-injected into the deep dermis and superficial hypodermis at the center of each square. The area is 10 cm × 10 cm around 64 squares for application.
Result: All patients have described an improvement in the area, with minimal adverse events. Ultrasonography images have shown an increase in the dermal thickness in the striae area.
Conclusion: PLLA application showed to be a good alternative to treat striae in abdominal and gluteal area.
Striae distensae; Cosmetic; Techniques; Poly-L-lactic acid; Diagnostic imaging
Striae Distensae (SD), commonly known as stretch marks is a common dermatological entity that affects patients of all ages, gender, and ethnic groups [1]. Although they do not pose any risks to health, they are considered aesthetically unacceptable with a significant psychosocial impact. Despite the countless number of treatments available, no proven efficacy with total lesion recovery has been observed so far, and for that SD represents a therapeutic challenge [2,3].
The first signs are linear edematous striations, ranging in color from red to violaceous (striae rubrae), which turn into white atrophic linear scars (striae alba) with the passing of time [4]. The highest incidence occurs during puberty and pregnancy with a prevalence ranging from 11% to 88% [1,3,5]. Among adolescents, the mean age for the onset is 13.8 years. In male adolescents, they are more frequently found on the outer side of the thighs and the lumbosacral region; in females, on the outer side of the thighs, gluteal region and breasts [1,3]. During pregnancy, they usually occur in the third trimester among 75%-99% of the patients [1]. Moreover, striae may also be associated with rapid weight gain or loss, complications with the use of topical or oral corticosteroids, a postoperative effect in breast augmentation procedures, an increase in muscle mass after intense physical exercises or the use of steroids and Cushing and Marfan syndrome [6].
Its physiopathology has not been totally clarified yet. So far, its etiology has been considered multifactorial: Hormonal influences associated with high levels of steroid hormones, which have catabolic effects on fibroblast activities, thus reducing the collagen deposition in the dermal matrix [1]; the mechanical stretching of the connective tissue due to the gain of weight or growth causes its rupture; reduction in the genetic expression of fibronectin, collagen and elastin [3,7]. Specifically, regarding the application of topical corticosteroids, a study using electron microscopy reveals that they lead to the inhibition of fibroblasts and the release of enzymes (elastase) by mast cells resulting in elastosis of the middle dermis [8].
Concerning histology, the alterations found depend on the type of striae. In cases of striae rubrae, a superficial and deep perivascular lymphocytic infiltrate can be observed well as the dilatation of venules and edema in the upper dermis. On the other hand, striae alba cannot be distinguished from a scar. The collagen fibers of the reticular dermis are elongated and aligned parallel to the epidermis, and there is an overall decrease in collagen and elastin [9].
The striae improving the aesthetic appearance is still challenging. Today, rather than total recovery, therapies aim to improve lesions, and better results can be observed in cases of recent striae. In such cases, treatments initially focus on the reduction of the vascularization and the erythema with Intense Pulsed Light (IPL) (ND-YAG LASER and Dye LASER) or the increase in collagen production, most often using procedures like the application of topical retinoids, chemical peelings, and microdermabrasion [6,10]. Mature striae are more resistant to treatments and improvements are less noticeable [11]. In general, the most indicated methods for the treatment of white striae are fractional LASER and micro needling [12-15].
The use of fractional techniques as the main forms of treatment for striae through collagen production stimulation not only leads to risks of hyperpigmentation and bad scar healing related to the phototype of the patient, but also imposes limitations when in the presence of important local flaccidity. The treatment challenge is to obtain results that correspond to the patient’s expectations with no relevant adverse effects, and not a partial improvement of the lesion as it is normally observed. Therefore, the therapeutic response is usually frustrating for both the physician and the patient.
Thus, since Poly-L-Lactic Acid (PLLA) induces a local and gradual reaction with the formation of new collagen by fibroblasts and a consequent increase in dermal thickness and that this fibroplasia will provide an improvement in skin flaccidity with great cosmetic results, its use has been considered in the treatment of white striae [16]. PLLA is an injectable biocompatible and biodegradable synthetic polymer from the alphahydroxy acid family with low toxicity. Injections of PLLA into the deep dermis or superficial hypodermis induce a local and gradual reaction that triggers the recovery of the collagen network lost during the aging process. Consequently, it may be useful in the collagen network alterations that occur during the striae formation process.
As the results may not be visible after each application for weeks, it is important to wait for the biological response between administrations. Additional treatment applications should occur with an interval of at least four weeks between each of them. Response time and improvement extent basically depend on the individual response of each patient, which varies according to age, gender, quality of the skin, phototype and dietary habits. Each application of PLLA will lead to the formation of collagen, whose magnitude will also depend on the concentration and volume applied [17,18].
Regarding safety of the PLLA, the first synthesis of PLLA was carried out by Carothers in 1932. They obtained a material with low molar mass and mechanical properties lower than the ones required for certain applications in medical fields. In 1954, Du Pont produced a polymer with a higher molar mass and patented it. However, its susceptibility to the reaction with water made researchers lose interest in it at that time. It was in 1966 that Kulkarni showed that the degradation of the material could happen in vivo, and from then on, a renewed interest in all fields of medicine has risen, especially in the production of surgical threads, stents and its use in microspheres as a vehicle for the release of drugs. Injectable PLLA has been applied as a cosmetic filler since 1999, and many studies confirming its safety, effectiveness and longevity have been published [19,20]. Sculptra®, with 150 mg of PLLA microparticles, 90 mg of sodium croscarmellose (CMC, for the dispersion of the particles) and 127.5 mg of non-pyrogenic mannitol (for a more rapid gel hydration), was the product of choice in this study. Thus, the aim of this study is to show improvement in the treatment of white striae with three monthly applications of poly-L-lactic acid in the affected regions.
This was a pilot study approved by the Institutional Ethics and Research Council Board and conducted in compliance with the Good Clinical Practice. A total of five patients were screened from the Cosmiatry Outpatient Clinic of the Department of Dermatology. They all presented with symmetrical and bilateral white striae, 1 mm-4 mm wide, located on the gluteal or abdominal region. Inclusion criteria were: Men or women aged between 20 and 40 years with white striae on the indicated regions who had stable weight over the period of the past six months and women should be on a contraceptive method. Patients who presented with any of the following characteristics: On a vegetarian diet, in a weight loss program, on corticoids or any other anti-inflammatory in the past six months, hypertension, metabolic disorders and diabetes, inflammatory diseases, skin conditions and allergies, pregnancy or lactating were excluded to the study.
The region to be treated was decided by the patient and the researcher. The importance of waiting for the biological response between each administration was discussed since the results are not visible for weeks after applications. All patients signed the informed consent.
The screened patients and investigator answered a questionnaire that included questions regarding the number and size of the striae prior to the beginning of the treatment. Pre-treatment photos were taken and a high-resolution ultrasound was performed in the region to be treated. The ultrasound images were carried out in the outpatient clinic using GE Logiq™ equipment (General Electric, USA) with a high frequency linear transducer of 22 MHz. Epidermis and dermis thicknesses, with and without striae, in those areas were measured, and the highest striae thickness values in each area were analyzed. Transversal and longitudinal sections of the skin were performed so that a better magnification of thickness measurements could be obtained. To evaluate the Striae, the researchers classified them according to the number of striae: (0) absence of striae; (1) up to 5 striae; (2) between 6 and 15 striae;(3) over 16 striae and the size (1) 1 mm-2 mm; (2) 3 mm-4 mm in at least 50% of the striae.
Product preparation process
The Sculptra® vial was reconstituted with 8 ml of sterile water 24 hours prior to use. Right before application, 2 ml of lidocaine at 2% with no vasoconstrictor along with 6 ml of sterile water were added, totaling a volume of 16 ml.
Application technique
A total of three sessions applications with 30 days between applications, were performed in the affected area 30 minutes after the application of 7% lidocaine/7% tetracaine cream. The skin area to be treated was demarcated with drawings of squares measuring about 1.5 cm2 each (Figure 1).
Figure 1: Demarcation of skin area to be treated with 1.5 cm2.
After a thorough cleaning of the area with chlorhexidine, a total of 8 ml of the solution was applied to each side. Using 1 ml syringes with 26G × 1 needle, 0.04 ml of the product was retro injected into the deep dermis and superficial hypodermis at the center of each square, totaling 12 ml according to the volume of preparation. At the end of the procedure, the treated area was massaged for about 5 minutes. Bruised areas were counted at the injection sites. Patients were advised to massage the treated area over 5 minutes twice a day for 7 days.
Evaluation
Evaluations were carried out by means of the following tools: (1) A questionnaire answered by the patient and the researcher after three and six months with questions concerning response to the treatment, adverse effects and occasional complications; (2) Photos of the area were taken before and after three and six months of the last application; (3) Ultra sonographic imaging before the treatment, three and six months after the last application were also done.
The improvement score established after six months was as shown in Table 1. The level of improvement was evaluated according to the questionnaire answers provided by the patients and researchers and through the photos analyzed by the researchers.
The final data-set comprised five patients aged between 20 and 40 (mean age 30 years), phototype I to IV according to the Fitzpatrick scale, with degrees of striae ranging grade 1 to 3. Six months after the PLLA application, patients’ and researchers’ evaluations in regard to the extent of improvement coincided. The scores obtained are shown in Table 1.
| Score | Improvement evaluation |
|---|---|
| 0 | 0%: No improvement |
| 1 | 1%-25%: Minor improvement |
| 2 | 26%-50%: Moderate improvement |
| 3 | 51%-75%: Great improvement |
| 4 | 76%-100%: Excellent improvement |
Note: Regarding adverse events, pain was classified as, (0) no pain; (1) mild pain; (3) moderate pain; (4) intense pain and the number of bruised areas after each application-(0) no bruises; (1) up to 3 areas; (2) from 4 to 7 areas; (3) more than 8 areas. Furthermore, ultrasound evaluation, skin thickness and organization of the dermal fibers distribution were also repeated after 6 months.
Table 1: Evaluation of striae improvement (a modified Blyumin-Harasik scale).
Clinical improvement was described and observed in all patients according to the questionnaire response (Table 2 and Figure 2), and a significant increase of dermal thickness as shown in Figures 3 and 4.
| Patient | Age | Gender | Phototype | Degree of striae | Pain | Bruising | Extent of improvement |
|---|---|---|---|---|---|---|---|
| 1 | 35 | F | IV | 3 | 1 | 0 | 2 |
| 2 | 34 | F | II | 2 | 1 | 1 | 2 |
| 3 | 26 | M | III | 1 | 1 | 0 | 3 |
| 4 | 30 | F | II | 3 | 1 | 1 | 3 |
| 5 | 20 | F | II | 2 | 1 | 1 | 3 |
Table 2: Patients’ data, improvement score and adverse effects according to the patients and researchers.
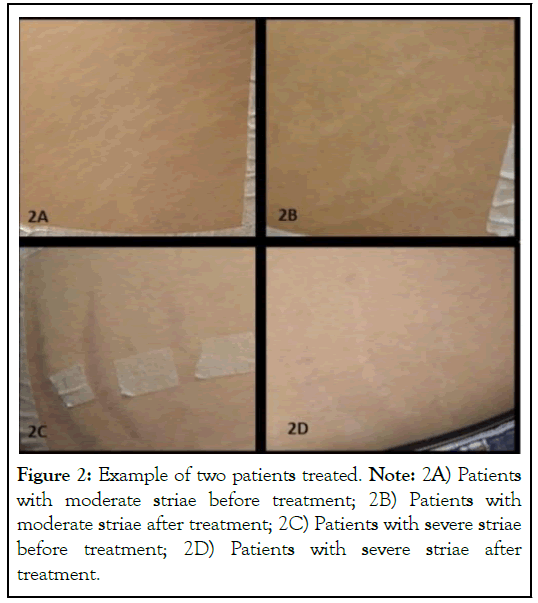
Figure 2: Example of two patients treated. Note: 2A) Patients with moderate striae before treatment; 2B) Patients with moderate striae after treatment; 2C) Patients with severe striae before treatment; 2D) Patients with severe striae after treatment.
Figure 3: Ultrasound images before and after 6 months of PLLA application. Circles and arrows show the dermal thickness. Note: 3A) Before ultrasound, 3B) After ultrasound.
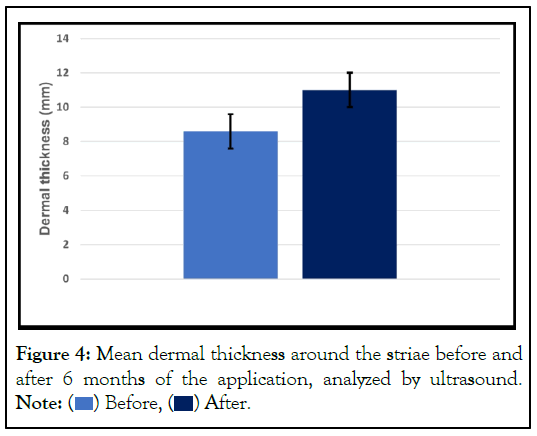
Figure 4: Mean dermal thickness around the striae before and
after 6 months of the application, analyzed by ultrasound.
Note:  Before,
Before,  After.
After.
Striae are intrinsically related to the stretching of the skin with the onset attributed not only to hormones, like those released during pregnancy and puberty and the use of oral or topical corticoids, but also to genetic predisposition [6]. The thinning of the dermal thickness observed in Striae Alba cases occurs due to structural and biochemical changes in elastic and collagen fibers as well as in the fundamental substance. There is an overall reduction in collagen amount and breaking down of collagen fibers resulting in elongated lines in the superior reticular dermis aligned in parallel to the epidermis. Elastin concentration is reduced, with a disorganized distribution of elastic fibers [9]. At palpation, thin and loose skin can be perceived. Such changes can be observed even in adolescent and young patients.
Many different approaches have been proposed for the treatment for improve aspect and appearance of Striae Alba; however, in many cases, results are discouraging. The mechanism of action of the micro particles of PLLA-SCA consists of the stimulation of fibroblasts as a subclinical tissue response to inflammation. The new collagen begins to form a month after the application and the amount continues to increase during a period ranging from nine months to one year. Therefore, your use in the treatment of striae was suggested, with evaluations during pre-treatment, three months and six months after the last administration so that a better analysis of the aesthetic injectable response of the product could be conducted. Its use for the treatment of body sagging has already been established with great but its application for the specific treatment of striae had not been described yet [16].
It is known that six months after the administration of PLLASCA, many particles become porous and they are surrounded by macrophages. Its degradation pathway occurs through nonenzymatic hydrolysis in lactic acid monomers, which are metabolized in CO2 and H2O or bonded to the glucose molecules. After approximately 18 months, it is completely eliminated by the body, a significant factor regarding the safety of the product in the treatment of striae, especially among young patients [20,21].
PLLA should not be injected in areas previously treated with permanent fillers, like silicone or polymethyl methacrylate. Its use is not approved in children, pregnant women or lactating mothers. Other contraindications include the use of immunosuppressant’s, heavy smoking and patients who expect immediate results. A different approach should be adopted with individuals who are on chronic use of immunosuppressant or anti-inflammatory drugs like prednisone since the response to the treatment will less effective than expected [20].
The improvement rate and the response to treatment were individual, regardless of age group, and non-proportional to the initial severity level, however all patients have shown an improvement the clinical aspects. Furthermore, ultrasound images have shown that the striae have hypoechoic echotexture (shiny dark gray) (Figures 3A and 3B), well identified in the images. At day 180, a decrease in width (thickness) of the striae as well as an increase in dermis thickness could be observed, confirming thus the clinical findings. The results were obtained by a High Frequency Ultrasound Scanning (HFUS) at a frequency ≥ 20 MHz which allows a precise evaluation and measurements of skin layers (epidermis, superficial dermis, papillary dermis and hypodermis).
The reported adverse events were related to the treatment related, the AE they were transitory, with no major impacts that could hinder of the treatment. No post inflammatory hyperchromia could be observed, even in the patient with a higher phototype, and none of the patients presented with delayed-onset nodules. All of them fulfilled the protocol with a great improvement of the striae according to the results obtained during the study period.
In the current study, suggest that PLLA-SCA injectable may be a good option for treating striae. There was an improvement in aspect in a short period of time with and well tolerated, that we can related to the application technique.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Cunha M (2023) Treatment for Striae Alba with Poly-l-lactic Acid: A Pilot Study. J Clin Exp Dermatol Res. 14:649.
Received: 25-Oct-2023, Manuscript No. JCEDR-23-27170; Editor assigned: 27-Oct-2023, Pre QC No. JCEDR-23-27170 (PQ); Reviewed: 13-Nov-2023, QC No. JCEDR-23-27170; Revised: 20-Nov-2023, Manuscript No. JCEDR-23-27170 (R); Published: 27-Nov-2023 , DOI: 10.35841/2155-9554.23.14.649
Copyright: © 2023 Cunha M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.