Journal of Genetic Syndromes & Gene Therapy
Open Access
ISSN: ISSN: 2157-7412
ISSN: ISSN: 2157-7412
Review Article - (2022)Volume 13, Issue 3
Every human disease undergoes many critical diagnostic methods to locate the reason behind the abnormality. About 50percent of deafness issues which account due to some genetic defects have been reported and 93 percent of these genetic deafness issues are monogenic autosomal recessive traits. DFNB is known for the majority of congenital deafness cases and it has different types based on varying loci. Among them, DFNB9, the autosomal recessive disorder, occurs due to the heterozygous mutation in OTOF gene (coding Otoferlin protein) present on chromosome 2p23. Today, the major advancement in the study of human deafness is the preparation of mouse models for different types of Nonsyndromic autosomal recessive deafness. Genetics has played a vital part in medicine because genomics study gives many critical hints about any abnormality in biological processes which may cause disease. This can be apprehended by the fact that proteins coded by genes play an essential role in the hearing process. With the advancement in treatment methods, gene therapy is the talk of the town today. AAV mediated gene therapy is considered as most promising technique nowadays. Adenovirus possesses many impressive qualities which make them efficient vectors to be used in the transduction of genes. The use of AAV has been reported for transducing hair cells as well as other cell types. Different experimentations have reported the delivery of AAV stereotypes to the neonatal mouse as a source of treating congenital hearing loss by targeting a wide range of chromosome loci.
Congenital deafness; Gene therapy; Chromosome 19; DFNB9; Dual AAV; Adenovirus
Deafness can be defined as the permanent or fluctuating loss of hearing ability. The main causes of deafness are regarded as environmental as well as genetic factors [1]. As reported by WHO in 2004, the disabling hearing obstruction was found in 5% of the world’s population, [2] 80% of those were due to genetic factors and 75%-80% of them indicated autosomal recessive inheritance accompanied by autosomal dominant in range of 20%-25% and 1%-1.5% of X linked or mitochondrial inheritance [3]. Hearing loss has been differentiated as Syndromic and Nonsyndromic hear loss.
Upto 30% of pre lingual deafness cases arise due to syndromic hear loss [4]. It describes the hearing impairment linked with some additional phenotypes including clinical issues mostly in the eye, kidney or, skin and occasionally in the musculoskeletal and nervous systems. Usher syndrome, Jarden and Lange- Nielson syndrome, and Wardenburg syndrome are the most common examples of syndromic hearing loss [5].
Nonsyndromic hearing loss (restricted to the inner ear) also referred to as sensorineural, has been recorded to prevail by 70% of total genetic hearing loss cases. Nonsyndromic has been further differentiated as ‘autosomal recessive’ and ‘autosomal dominant’, on the basis of their mode of inheritance. Autosomal recessive hearing loss is mostly labeled as DFNB which occurs by 80% of all autosomal cases and the remaining 20% are autosomal dominant labeled as (DFNA) [6] (Figure 1).
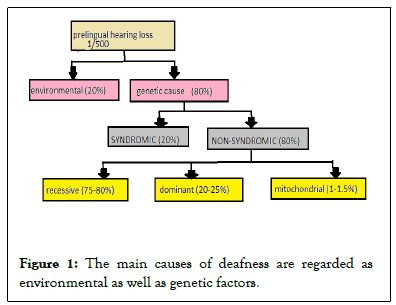
Figure 1: The main causes of deafness are regarded as environmental as well as genetic factors.
Molecular genetics basis of non-sydromic deafness
The first nuclear gene responsible for Nonsyndromic deafness was isolated for the first time in 1995. Later on, further molecular research in field of genetic deafness lead to the finding of 60 loci and 25 genes involved in congenital hearing impairment [7]. Nonsyndromic deafness is classified as Autosomal dominant and autosomal non dominant deafness. Nonsyndromic Autosomal recessive refers to the alteration in both copies of the gene. This concept conveys that both the parents carry recessive deafness genes which are inherited in the next generation thus phenotype of autosomal recessive deafness appears. While the autosomal dominant pattern of Nonsyndromic deafness gives the altered genetic basis in at least one of the copies of gene capable of developing deafness in the next generation of the parent having deafness trait [8].
Nonsyndromic autosomal recessive deafness genes
About 23 autosomal recessive genes have been identified to be responsible for 80% of Nonsyndromic deafness cases with prelingual onset. The heterogeneity of DFNB genes implicates the wide range of functions played in the development of deafness. It has been reported that mutation in the GJB2 gene is responsible for 50% of autosomal recessive deafness cases having pre-lingual onset. While SLC26A4, MYO15A, OTOF, TMC1, CDH23, and TMPRSS3 are the other most common recessive deafness genes, OTOF being on locus DFNB1, [4]. The most common loci for recessive deafness include DFNB1, DFNB2 and DFNB3. This recessive trait is not only limited to these loci but is also extended to many other genes loci [9].
Chromosome21 also harbors the disease locus DFNB8 to cause recessive deafness [10]. In the Pakistani population, the most common mutations resulting in Nonsyndromic congenital deafness occur in GJB2 resulting in 6.1%–53% of genetic hearing loss issues. Other known loci contributing in genetic deafness are, OTOF (2.3%), TMIE (1.7%), SLC26A4 (7.2%), TMC1 (3.4%–5.4%), MYO15A (3.3%–13%), and TMPRSS3 (1.8%–2.5%) [11].
Gene therapy technique for congenital deafness
Today many research projects are in the process to make molecular medicine the most practical way to cure many inherited diseases. In this aspect, gene therapy is the talk of the town in an area of treating inherited diseases, which works on a basic idea of transferring gene in a tissue, cell or organ to cure a disease or to clinically reduce the progression of disease [12]. Major medical progress has been made to treat a wide range of diseases including immunological disorders, heart diseases, and cancers by setting emerging field of gene therapy in action. This goal has been achieved by developing proper transducing vehicles or vectors, thus, a gene delivery system is the crucial step in gene therapy [13].
The important vector which has been emerged for gene therapy is Adeno-Associated virus. There are many advantages that make AAV capable of gene therapy including long-term gene expression and decreased tendency to cause pathogenicity from wild infections. Moreover, it requires a helper virus to replicate and facilitates transduction of dividing and non-dividing cells. The utilization of AAV for gene therapy has gone through a number of Phase I and Phase II clinical trials [14,15].
Biology of adeno-associated virus
Adeno-Associated Virus (AAV) is a member of the family Parvoviridae. Members of this family are small, nonenveloped, icosahedral viruses with a diameter ranging from 20–26 nm. They possess genomes of 4.7–6 kb with liner, single-stranded DNA containing 4679 nucleotides. The genus Dependovirus has been assigned to AAV. Dependovirus is so named due to the inability of the virus to infect cells in culture without coinfection by an unrelated helper virus, which is adenovirus most often, but it also can be any type of herpes virus. It was a misconception about AAV that it is a defective virus due to its need for a helper virus to cause a notable infection in cell culture but it has been demonstrated by some studies that the latent infection can be produced in a healthy cell by this virus and also the stress condition can induce its vegetative part [16].
Adeno-associted AAV as vector
The reasons, for which Adeno-Assosiated Virus proved to be an efficient vector for replacing genes with complementary DNA having coding sequences less than 4 KB, in inner ear cells, are their non-pathogenicity. Also, among all viral vectors discovered till today, AAV is the less immunogenic. Moreover, it has been found that various inner ear cells working as major contributors in causing different types of genetic hearing loss (inner and outer hair cells) have been transduced by some engineered as well as natural capsid proteins [17]. However, there is one limitation about using AAV as vectors is their limited cargo capacity of foreign DNA with coding sequences up to <4 kb only. To get over this limitation dual AAV approaches are being used for transducing large molecular weight foreign DNA [18].
Dual AAV strategies to develop dual AAV vector
The reconstitution of larger genes in target cells has been successfully operated by various dual AAV strategies. It involves some processes based on various approaches named (1) Overlapping, (2) Trans-splicing, and (3) Hybrid AAV vectors.
Overlapping approach: The genome can be reconstituted in full length by using an overlapping approach. In this approach, coding sequences of both AAV vectors use the concept of homologous recombination of CDS overlapping sequences. Thus, the promoter is carried by the 5’vector and also half of the 5′of coding sequence. The 3′-vector carries the 3′ half of the coding sequence as well as the polyA signal [19].
Trans-splicing approach: In this approach, the genomes can be reconstituted in their full length by using the characteristic feature of the Adeno-Associated virus’s Inverted Terminal Repeats (ITRs) to undergo concatemerization. Using this approach, the transgene is carried in two halves by two vectors. They are designed in a way that the promoter is possessed by 5’vector. The AAV genome has a Splicing Donor (SD) signal at the 3’end of the 5’-half vector while it possesses a splice acceptor signal at the 5’end of the genome in the 3’-half vector. The genomes of two AAVs are concatemerized through tail-to-head for the reconstitution of the genome in full length, the concatemerized ITR structure is spliced through the SD and SA signal forming the junction point so that the intact large mRNA molecule is obtained.
Hybrid approach: This approach includes the combination of concepts of trans-splicing and overlapping approaches. This technique enhances the recombination efficacy by loading the trans-splicing vectors with exogenous sequences having high recombination power [20] (Figure 2).
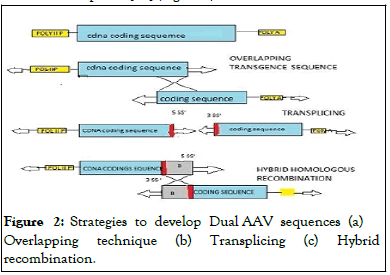
Figure 2: Strategies to develop Dual AAV sequences (a) Overlapping technique (b) Transplicing (c) Hybrid recombination.
Major contributions
Akile, et al. reported an experiment performed on anesthetized OTOF deficient mouse in which AAV Otof vector pair was delivered through the round window cochlea. Remarkably, when this local gene therapy was applied before onset of hearing ability, it prevented the deafness in OTOF−/ mice as well as maintained the deafness phenotype administered before hearing onset. The recorded data of results recommends therapeutic operation in DFNB9 mutant patients suffering with congenital deafness [21]. The mutation in connexin26, the gap junction protein, related GJB2 gene (Cx26) causes Nonsyndromic congenital deafness in humans due to the absence of gap junction protein connexin 26. This protein expression is Yu, et al. controlled by the GJB2 gene while due to mutations in this gene results in deafness.
Yu, et al. has reported the use of AAV viral vectors to restore the C26 expression in cochlear tissues of the mutant mouse by recovering the GJ mediated coupling. Cochlear GJ network was functionally recovered as well as phenotypic improvement was also observed in the injected cochlea. These results proved remarkable for removing GJB2 mutation by gene therapy for treating Nonsyndromic deafness [22]. Györgyet, et al. have reported the complete inner and outer hair cells transduction by AAV9-PHP.B in an Usher syndrome type 3A deafness (gene CLRN1) mouse model [23].
The past 5 years have been so productive to take appreciating steps in Cochlear gene therapy. The first research in this aspect included the use of AAV-mediated gene therapy for restoring congenital hearing loss in a mouse lacking a VGLUT transporter, which proved to be successful and was documented for future clinical application of this methodology. The hearing ability of the VGLUT3 knockout mice model was restored using AAV as a vector for genes transduction in the inner ear.
The authors declare that there is no conflict of interest.
[Crossref], [Google Scholar]
[Crossref], [Google Scholar]
Citation: Fatima M (2022) Treatment of Autosomal Nonsyndromic Congenital Deafness by AAV Mediated Gene Therapy. J Genet Syndr Gene Ther. 13:364.
Received: 15-Apr-2022, Manuscript No. JGSGT-22-15571; Editor assigned: 22-Apr-2022, Pre QC No. JGSGT-22-15571 (PQ); Reviewed: 06-May-2022, QC No. JGSGT-22-15571; Revised: 13-May-2022, Manuscript No. JGSGT-22-15571 (R); Published: 20-May-2022 , DOI: 10.35248/2161-0517.22.13.364
Copyright: 2022 Fatima M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.