Virology & Mycology
Open Access
ISSN: 2161-0517
ISSN: 2161-0517
Research Article - (2022)Volume 11, Issue 1
In vitro and preclinical studies have demonstrated better therapeutic efficacy with eberconazole than clotrimazole and ketoconazole. The objective of current investigation was to study the degradation behavior of eberconazole nitrate and mometasone furoate under different International Conference on harmonization recommended stress condition using reverse phase high performance liquid chromatographic method and to establish validated stability indicating high performance liquid chromatographic method to determine purity of eberconazole nitrate and mometasone furoate in presence of its impurities, forced degradation products and placebo in pharmaceutical dosage forms. Topical application of eberconazole was well tolerated in preclinical studies without any report of delayed hypersensitivity or photosensitivity reactions. There were no phototoxic effects. There was no significant systemic absorption. Animal toxicity studies have shown that it is safe, and the No Observed Effect Level was 2 ml/kg body weight in tested animals. It was not mutagenic and shared similar cytotoxicity profile with other imidazole antifungal products studied The method was developed using Persil BDS, C18, 150 × 4.6 mm, 5 μ as stationary phase with mobile phase containing a gradient mixture of solvent A and B. 0.01 M phosphate buffer with 0.1% triethyl amine, adjusted pH 7.0 with phosphoric acid was used as buffer. Buffer pH 7.0 was used as solvent A and methanol: acetonitrile in 150:850 v/v ratios were used as solvent B. The eluted compounds were monitored at 240 nm. The run time was 50 minutes. The developed method was validated as per international conference on harmonization guidelines with respect to specificity, linearity, limit of detection, limit of quantification, accuracy, precision and robustness.
Cream formulation; Ointment; Lotion; Eberconazole nitrate; Mometasone furoate
Eberconazole nitrate and mometasone furoate in cream formulation (topical antifungal and ant parasites) is the combination of eberconazole nitrate a topical imidazole derivative, which has shown high potency against dermatophytes and yeasts (several species of Candida, Malassezia) In vitro and in experimental models. Eberconazole nitrate is described chemically as: 1-(2,4-dichloro-10,11- dihydro-5Hdibenzo[ a,d]cyclohepten-5-yl)-imidazole Nitrate. Its empirical formula is C18 H14 Cl2 N2 and molecular weight is 329.22+63.01. Mometasone furoate is a synthetic corticosteroid with anti-inflammatory activity. Chemically, Mometasone furoate is (11β,16α)-9,21-Dichloro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methyl-pregna-1,4-diene-3,20-dione. Its molecular formula is C27 H30 Cl2 O6 and molecular weight is 521.44. Each 1 g of cream contains 1% w/v eberconazole nitrate and 0.1% w/v mometasone furoate. Methods are available for determination of mometasone furoate and its degradation in human plasma [1]. Mometasone furoate assay by UV detection methods are also available [2]. Supercritical fluid chromatography detection methods are also available for mometasone furoate. So far to our present knowledge, no validated stability indicating analytical High Performance Liquid Chromatography (HPLC) method was available in literature for mometasone furoate and eberconazole nitrate and its impurities Figure 1 in cream formulation. Attempts were made to develop a stability indicating LC method for the related substance determination of eberconazole nitrate and mometasone furoate in cream formulation. This paper deals with the validation of the developed method for the accurate quantification of eberconazole nitrate and mometasone furoate impurities in cream formulation.
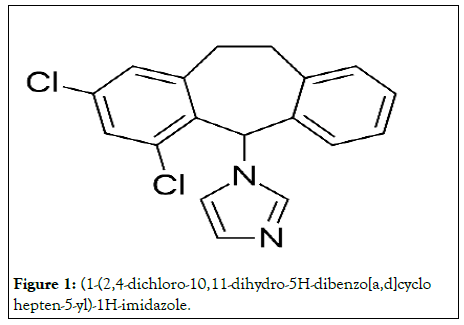
Figure 1: (1-(2,4-dichloro-10,11-dihydro-5H-dibenzo[a,d]cyclo hepten-5-yl)-1H-imidazole.
Structure
Mechanism of action: Eberconazole exerts fungicidal or fungi static activity depending on concentration, being fungicidal at higher concentration and fungi static at lower concentrations. Eberconazole inhibits fungal growth by inhibiting ergo-sterol synthesis, an essential component of the fungal cytoplasmic membrane leading to structural and functional changes. It inhibits the fungal ergo sterol synthesis by inhibiting lano-sterol 14α-demethylase enzyme that is responsible for the formation of 14 α-methyl sterols (precursor of ergo sterols) [3]. Studies have shown that eberconazole binds to the phospholipid fraction of the cell and affects sterol synthesis intracellular. At high concentrations, it causes the leakage of small molecules such as potassium ions, amino acids, inorganic phosphate and nucleotides from the fungal cell leading to cell death. The antiinflammatory activity comparable to acetyl salicylic acid and ketoprofen [4] shown in vivo by eberconazole is attributable to the inhibition of 5-lipooxygenase and to a lesser extent of cyclooxygenase-2.
Molecular formula: C18 H14 Cl2 N2 , is a broad-spectrum antifungal agent.
Molecular weight: 705.64 g/mol.
Spectrum of activity
Eberconazole has been shown to have broad antimicrobial spectrum of activity In vitro, to be effective in dermatophytosis, candidiasis, and infection by other yeasts such as Malassezia furfur and causative agents of Pityriasis versicolor in In vitro and animal studies. Its effectiveness against most diazole-resistant yeasts (Candida krusei and Candida globate) [5] and also fluconazole resistant Candida albicans has been demonstrated in vitro. It has also been shown to be effective against Gram-positive bacteria. Eberconazole is distinct from other imidazoles as it has been to shown to have anti-inflammatory activity, which favors its use in the management of inflamed dermatophytes infections.
Local toxicity of eberconazole
It was non-irritant in the primary ocular tolerance test with index of acute ocular irritation of 1.33. Local toxicity of eberconazole cream was compared to bifonazole in albino rats. Both produced similar degree of irritation, but eberconazole found to be slightly lesser irritant compared to bifonazole. It was non-irritant to vaginal mucosa of New Zealand rabbits. No allergy or hypersensitivity was observed as Buehler test was negative in guinea pigs. It shared similar cytotoxicity profile with other imidazole antifungal products studied i.e. miconazole, econazole, and clotrimazole when studied using BALB/3T3 cell line. Skin reaction score was measured using eberconazole 1% w/w lotion in Sprague Dawley rats (method of Draize) (Tables 1 and 2).
| Eberconazole cream 1% w/w | Clotrimazole cream 1% w/w | |||
|---|---|---|---|---|
| µg/cm2 released | µg/cm2 released | |||
| Time (h) | Set 1 | Set 2 | Set 1 | Set 2 |
| 0.5 | 1.3 | 1.8 | 1.2 | 0.5 |
| 1 | 3.6 | 4 | 2.9 | 1.1 |
| 2 | 6.9 | 7.2 | 5 | 2.1 |
| 4 | 10.6 | 11.6 | 6.5 | 3.6 |
| 6 | 16.3 | 15.9 | 7.9 | 5.3 |
Table 1: Comparison of penetration of Eberconazole 1% cream and Clotrimazole 1% cream.
| Eberconazole cream 1% w/w | Clotrimazole cream 1% w/w | |||
|---|---|---|---|---|
| µg/cm2 released | µg/cm2 released | |||
| Time (h) | Set 1 | Set 2 | Set 1 | Set 2 |
| 0.5 | 1.6 | 1.7 | 0.6 | 0.3 |
| 1 | 3.6 | 3.7 | 1.3 | 0.8 |
| 2 | 6.4 | 6.7 | 2.6 | 2 |
| 4 | 10.2 | 11 | 4.8 | 4 |
| 6 | 14.1 | 15.1 | 7.2 | 6.7 |
| Flux | 2.35 | 2.52 | 1.21 | 1.12 |
Table 2: Comparison of penetration of eberconazole 1% cream and terbinafine1% cream.
In vitro studies
Torres compared the In vitro activity of eberconazole with that of clotrimazole, ketoconazole and miconazole against 200 strains of dermatophytes belonging to 19 species of fungi. Among the four drugs tested, eberconazole exhibited the lowest MIC for majority of the dermatophytic strains (p<0.05), suggesting an advantage of eberconazole over other widely used agents [9].
In another In vitro study, eberconazole was shown to be as efficacious as or even better than clotrimazole and ketoconazole against various strains of Candida especially C. krusei and C. glabrata, which are usually resistant to triazoles.
Preclinical studies
In preclinical studies using experimental models of superficial fungal infections, eberconazole has been shown to exhibit similar efficacy to clotrimazole, ketoconazole and better efficacy than bifonazole. The studies showed that eberconazole is well tolerated without any delayed hypersensitivity or photosensitivity reactions. Also, no phototoxic effects were seen and no significant systemic absorption was observed with eberconazole [6].
Human pharmacokinetics
After topical application of eberconazole 2% cream in healthy volunteers, its concentration in human plasma and urine were below the lower limits of detection (<1.1 ng/ml for plasma and <1.0 ng/ml for urine) when analyzed by high performance liquid chromatography. However, data on metabolism and excretion of topical eberconazole are not available.
Efficacy
Efficacy and safety of topical eberconazole have been established by a number of studies. Twice daily application of eberconazole 1% was found to be as efficacious as eberconazole 2% in 60 patients with mycological proven Tinea corporis and Tinea cruris, in a phase II pilot study, but the measured parameters did not show any statistical significance. Overall clinical cure rate post therapy was 73.3-93.3% in different groups. Adverse effects were seen more with eberconazole 2% than with 1%, but without any statistical significance. Further, efficacy of eberconazole has been compared with the widely used topical antifungals in various studies. In a double blind study, eberconazole 1% cream′s therapeutic efficacy and safety profile was similar to miconazole. After 4 weeks of therapy, 76.09% patients on eberconazole showed effective response compared to 75.0% in miconazole group Therapeutic effects of eberconazole with clotrimazole and miconazole are compared. In another multicentric double blind randomized study, compared the efficacy of eberconazole 1% cream with miconazole 2% cream in the treatment of dermatophytes. In this study, eberconazole showed similar efficacy and safety profile with miconazole in the treatment of dermatophytes infection as presented in Table 3. This study also suggested that eberconazole can be considered a good alternative for the treatment of dermatophytes as it has good safety and tolerability profile [7].
| Ingredients | |
|---|---|
| Eberconazole nitrate Ip | Quantity |
| Eq to eberconazole | 1.0%w/w |
| Cream base Ip | Q.S |
| Propyl paraben Ip | 0.1%w/w |
| Preservative | |
| Methly paranean Ip | 0.1%w/w |
| Propyl paraben Ip | 0.025%w/w |
Table 3: Formulation of eberconzole.
The results of study in Indian patients has confirmed the earlier findings that eberconazole 1% is significantly effective against cutaneous dermatomycoses and the reported AEs were similar to that seen in other studies indicating that it is well tolerated in majority of patients. Data, available on eberconazole, is quite limited. Data on its metabolism and excretion are still not available thereby requiring further studies. Although it is effective against triazole resistant yeasts, it is found to be less effective clinically compared to clotrimazole 1% thereby limiting its use in this condition. Moreover, comparative efficacy is yet to be established with allylamines and other new antifungal agents. The rise in fungal infections due to increase in incidence of immune compromised states, change in socioeconomic and cultural states is demanding an effective antimitotic agent for the treatment and cure. Superficial fungal infections being more prevalent in tropical countries, an antimitotic agent having good safety profile and better efficacy with less chance of developing drug resistance such as eberconazole is always welcome. With newer antifungal agents flooding the market, eberconazole effectiveness in the treatment of a wide range of cutaneous fungal infection in comparison with the existing and newer agents has to be established to determine its place in the topical therapy of fungal infections in future [8,9].
Eberconazole broad spectrum of anti-mycotic activity against yeast and fungi, high efficacy in the preclinical studies led to its clinical development to explore safety and efficacy in humans. Its anti-inflammatory action and efficacy against gram positive bacteria can add to its efficacy in inflamed cutaneous mycoses and in secondary infections, favoring the regression of inflammatory symptoms and treatment compliance. Eberconazole is clinically effective in the treatment of topical fungal infections, with a good safety profile and good tolerability. It has acceptable topical availability with no detectable systemic drug levels, and does not appear to cause skin sensitivity [10].
Eberconazole was more active In vitro against a broad range of dermatophytes species than the other topical drugs tested suggesting that it may be a good alternative for the topical treatment of dermatophytoses. Various clinical studies have documented its efficacy in the treatment of dermatophytosis. Eberconazole showed greater therapeutic efficacy than clotrimazole 1% and equivalent efficacy with miconozole 2% in the management of dermatophytosis and was similar in efficacy in the management of cutaneous candidiasis and Pityriasis versicolor.
The need for newer antifungals has been propelled by the complexities mentioned above in the treatment of dermatomycosis. Eberconazole with its efficacy and safety profile finds an important place amongst the commonly used antifungals, and scores above similar molecules, being patientfriendly and dermatologist-prescribed. Complexities in the treatment of dermatomycosis have compelled the invention of newer antifungal agents with better efficacy and safety profile. Proven better efficacy, good safety and tolerability profile of eberconazole along with lack of sensitizing ability make it an attractive and suitable alternative in the management of dermatomycosis. However, further comparative studies with other commonly used agents are required to position eberconazole among topical antifungals. Complexities in the treatment of dermatomycosis have compelled the invention of newer antifungal agents with better efficacy and safety profile. Proven better efficacy, good safety and tolerability profile of eberconazole along with lack of sensitizing ability make it an attractive and suitable alternative in the management of dermatomycosis. However, further comparative studies with other commonly used agents are required to position eberconazole among topical antifungals.
[Crossref], [Google Scholar]
[Crossref], [Google Scholar]
[Crossref], [Google Scholar]
[Crossref], [Google Scholar]
[Crossref], [Google Scholar]
[Crossref], [Google Scholar]
[Crossref], [Google Scholar]
Citation: Krushna G, Rajendra SKS (2022) Treatment of Super Facial Mycoses Research: Ebercanozole /Ebernet. Virol Mycol. 11: 221.
Received: 30-Dec-2021, Manuscript No. VMID-21-41693; Editor assigned: 01-Jan-2022, Pre QC No. VMID-21-41693 (PQ); Reviewed: 15-Jan-2022, QC No. VMID-21-41693; Revised: 19-Jan-2022, Manuscript No. VMID-21-41693 (R); Published: 26-Jan-2022 , DOI: 10.35841/2161-0517.22.11.221
Copyright: © 2022 Krushna G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.