
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Mini Review - (2021)Volume 12, Issue 8
The radionuclide 117mSn (tin-117m) embedded in a homogeneous colloid is a novel Radio Synovi Orthesis (RSO) device for Intra-Articular (IA) administration to treat synovial inflammation and mitigate Osteo Arthritis (OA) in dogs. A study to evaluate tin-117m colloid treatment response in dogs with OA was conducted at two centers, the School of Veterinary Medicine at Louisiana State University, and at a referral practice in Houston, Texas. The tin-117m colloid was administered per-protocol to 14 client-owned dogs with radio graphically confirmed grade 3 OA in one or both elbow joints. Dog owners and attending clinicians assessed the level of pain at Base Line (BL) and the posttreatment pain response at 90-day intervals for one year. Owners assessed treatment response according to a Pain Severity Score (PSS) and a Pain Interference Score (PIS) as defined by the Canine Brief Pain Inventory. Clinicians reported a lameness score using a 0-5 scale, from no lameness to continuous non-weight bearing lameness, when observing dogs at a walk and a trot. The rate of treatment success as determined by improved mean PSS and PIS scores reported by dog owners was >70% at all-time points. Clinicians reported an improved mean pain score from BL at post-treatment Days 90 (p<0.05), 180, and 270. The dog owner and clinician assessments of treatment success were significantly correlated (p>0.05) at Day 90 and Day 180 time points. Results indicated that a single IA dose of tin-117m colloid provided a significant reduction in pain and lameness and improved functionality for up to a full year, with no adverse treatment related effects, in a high percentage of dogs with advanced, clinical OA of the elbow joint.
Inflammation; Aging; Cytokines; Delirium; Dementia; Conditional logic; Boolean
Osteo Arthritis (OA) affects ~20% of adult dogs and is increasingly understood to be a vicious cycle [1]. Recognizing this can open up new methods of treating this complex condition [1]. OA has traditionally been viewed as a cartilage-only disease; however, synovitis (i.e., inflammation of the synovium) is a common clinical finding [2]. Clinical signs of inflammation, histologic inflammation in osteoarthritic synovial tissue, and early cartilage lesions at the border of the inflamed synovium strongly indicate that synovitis plays a pivotal factor in the pathogenesis of OA [3]. Synovial inflammation is implicated in many signs of OA, including joint swelling and effusion. The OA synovium has both inflammatory and destructive responses that depend largely on macrophages. These effects are cytokinedriven primarily through a combination of interleukin-1 and tumor-necrosing factor [4].
Such observations are stimulating increased investigation into dynamic changes within the microenvironment of the synovial joint. The goal of such research is to develop therapies that can decrease both inflammatory synovitis and the production of degradative enzymes, which contribute significantly to the progression of OA [4]. With this goal in mind a new focus for chronic pain management is the macrophage, which acts as the conductor of the inflammatory orchestra. Inflammation, pain, impaired mobility and function, and structural changes characterize OA and contribute to its progression [5-7].
Pain is the hallmark of OA and results in both local and distant deterioration of the musculoskeletal system as a result of decreased and altered mobility. The pathologic process of OA, including joint capsule thickening and periosteal reactions, causes an altered range of motion, compounding musculoskeletal changes. Continual nociceptive input in the CNS results in somatosensory system deterioration and central sensitization with wind-up, amplifying the perception of pain [8]. Presently, the functional and structural changes associated with canine OA are incurable. Early intervention has the greatest potential for providing the most effective management of OA by providing the opportunity to initiate an appropriate long-term care plan and to disrupt the progressive, vicious cycle of multidimensional deterioration involving both the neurologic and musculoskeletal systems. From such recognition has come a proposed instrument for staging canine OA, the Canine Osteo Arthritis Staging Tool [9]. This tool can help identify OA at an early stage, noting pre-radiographic changes and improving dog owners’ awareness of early-stage OA. Clinician/pet owner synergism can help drive earlier and, therefore, more successful treatment by slowing disease progression.
Synovetin OA is a groundbreaking, new treatment that provides durable relief from the chronic pain and inflammation of canine elbow OA. With 1 fast intra-articular injection, effects last up to 1 full year, providing veterinarians and pet owners with a convenient approach to managing chronic pain and inflammation. By depleting proinflammatory macrophages (the source of chronic pain), Synovetin OA breaks the vicious cycle of inflammation, providing effective, long-lasting, safe, nonsystemic relief. During 3 separate yearlong clinical trials involving 69 client owned dogs, Synovetin OA was shown to be safe and effective [10]. One of these studies also showed Synovetin OA to be safe for re-administration to a previously treated elbow joint. Radiosynoviorthesis (the restoration of the synovium using a radioisotope) is a well-established human procedure, having been used around the world for >60 years, and is associated with a low adverse event rate (0.013%) [11]. Arthritic joints, which contain proinflammatory macrophages recruited during synovial hyperplasia, engulf colloid-embedded tin-117m microparticles, the active agent in Synovetin OA, and transport this complex to areas of synovial inflammation. Thereafter, tin-117m conversion electrons destroy the engorged macrophages responsible for inflammation via the no inflammatory process of apoptosis along with other synovial cells within 300 microns of the micro particles therapeutic radiation. This results in the synovium more closely reflecting the pre-inflammatory state and retards nociceptive transduction (pain). After decay, residual microparticles of inert tin are cleared via the lymphatic system to the liver.
Homogeneous Sn-117m Tin colloid (HTC) is a novel veterinary device developed as an intra-articular radiotherapy of inflamed joints via Radio Synovi Orthesis (RSO). HTC consists of highly insoluble Sn-117m Tin hydroxide with a controlled particle size, in the form of a colloid suspension. The mechanism-of-action is the delivery of ionizing radiation (conversion electrons) to inflammatory cells, located within the synovium, that are responsible for joint inflammation, pain, loss of function and structure. The therapeutic effects on the synovium do not rely on pharmacological means and remain fixed within the joint until after Sn-117m has decayed to background levels. Sn-117m decays by electron capture with emission of short range (0.22-0.29 mm) low energy conversion electrons with therapeutic application. Compared to types of beta emission, which produce a range of tissue penetration per emission energy, conversion electrons show more discrete energies and well-defined tissue penetration depths. In addition, the prolonged low energy irradiation of the synovium produces minimal fibrosis and minimizes synovial scarring.
Synovetin OA consists of a homogeneous tin colloid which emits a discrete (<300 μm) low energy conversion electron radiation within the joint space. The colloid is composed of micronized particles (1.5 μm to 20 μm) in which 99% have been shown to be retained in the joint space of the dog for at least 42 days (3 half-lives). The particles are absorbed and retained by synoviocytes and macrophages in the synovium, resulting in apoptosis and non-inflammatory ablation of inflammatory and inflamed cells. Ablation of the inflammatory cells reduces inflammation of the joint synovium. The mode of action is radioactive decay and is intended to reduce synovitis and associated pain and inflammation of joints afflicted with osteoarthritis (Figure 1).
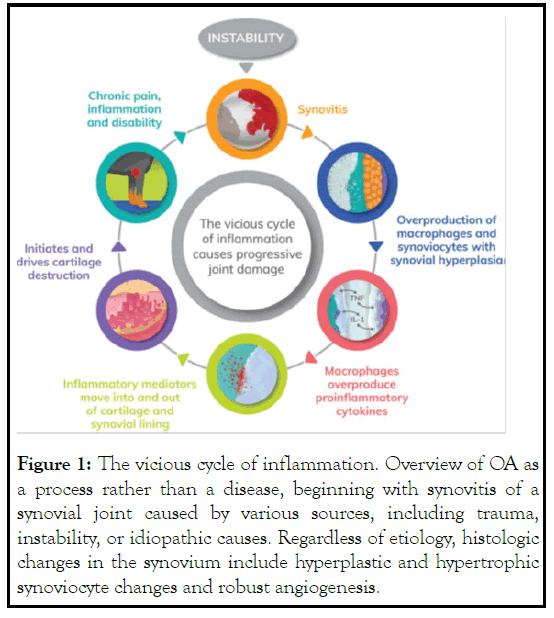
Figure 1: The vicious cycle of inflammation. Overview of OA as a process rather than a disease, beginning with synovitis of a synovial joint caused by various sources, including trauma, instability, or idiopathic causes. Regardless of etiology, histologic changes in the synovium include hyperplastic and hypertrophic synoviocyte changes and robust angiogenesis.
Mostly macrophages, and these changes cause an overproduction of pro-inflammatory cytokines (eg, matrix metalloproteinases, interleukins, tumor-necrosing factor) that become part of the joint fluid milieu moving to and fro synovial and cartilage tissues as the dog loads and unload the joint. Matrix metalloproteinases, aggrecanases (metalloproteinase which cleave the aggrecan building blocks of cartilage), and nitric oxide, an important mediator in chondrocyte apoptosis, are particularly destructive to cartilage. With cartilage catabolism comes progressive inflammation, pain, and disability, contributing to the vicious cycle.
To date, no agent has been shown to have disease modifying effects on the structural progression of OA. Current therapies, including nonsteroidal anti-inflammatory drugs, COX-2 selective agents, piprants, Intra-articular hyaluronic acid injections, and opioids offer only symptomatic relief. Agents that have demonstrated potential efficacy for disease modification include the MMP inhibitor doxycycline and the combined lipoxygenase/cyclooxygenase inhibitor licofelone [12,13]. Despite the clear role of inflammation in OA, recent trials of potent anti-inflammatory therapies, including use of systemic and intra-articular biologic agents to inhibit TNFα and IL-1β, proved disappointing [14]. Major challenges for the development of Disease Modifying Osteoarthritic Drugs (DMOADs) include the need for improved measures of structural damage (beyond the use of X-rays) as well as improved understanding of the appropriate population and disease stage for intervention. OA is a final common pathway following many predisposing factors and thus therapeutics may have limited, if any, efficacy in those with pre-existing joint damage, biomechanical predisposition, or obesity [15]. The requirement of radiographic change, a finding observed in those with relatively advanced OA, likely identifies a population less amenable to anti-inflammatory intervention. No studies to date have targeted very early OA at a time when anti-inflammatory intervention might be most effective. Although synovitis is more frequently observed in those with end stage OA, the ability to identify and quantitate synovitis (and vis à vis inflammation) before the onset of irreversible joint failure, provides great promise to the targeting of pre-radiographic inflammation akin to the paradigm now well accepted for the early aggressive treatment of RA [16,17].
Thus, the increasing appreciation of clinical risk factors for the development of OA, as well as the advent of highly sensitive imaging modalities capable of visualizing early synovitis and cartilage change, holds great promise for the identification of the at-risk population most suitable for very early antiinflammatory interventions.
There are no conflicts of interest.
Citation: Donecker J, Fabiani M, Lorrie C, Aulakh KS (2021) Treatment Response in Dogs Withnaturally Occurring Grade 3 Elbow Osteoarthritis Following Intra-Articular Injection of 117mSn (Tin) Colloid: A Review. J Anesth Clin Res. 12: 1019.
Received: 02-Aug-2021 Accepted: 17-Aug-2021 Published: 24-Aug-2021 , DOI: 10.35248/2155-6148.21.12.1019
Copyright: © 2021 Donecker J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.