Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research Article - (2021)Volume 10, Issue 2
Background: Malaria, a common and life-threatening disease in many tropical and subtropical areas, caused by infection of red blood cells with protozoan parasites of the genus plasmodium inoculated into the human host by a feeding female anopheline mosquito. Malaria is a major public health problem in Ethiopia and has been consistently reported as one of the three leading top causes of morbidity and mortality. In East Wollega Zone there is lack of empirical evidences on the level of malaria prevalence.
Material and Methods: A retrospective study was carried out to determine the two year (July 2018 to June 2020) malaria prevalence based on district health information system version two (dhis2) database reports. All malaria cases reported in the specified periods were carefully reviewed by using questionnaire and analysed. Data were entered, processed and analysed into Microsoft Excel 2010 and then imported into Statistical Package for the Social Sciences (SPSS) version 24. Different graphs and tables were used to present trends of malaria cases and total population.
Results: Between July 2018 to June 2018, a total of 125,917 suspected malaria cases examined from all districts of East Wollega Zone and 26,679(21.2%) confirmed malaria cases were reported. Malaria was reported in both sexes and all age groups, but males (14802, 55.5%), and age groups ≥ 15years (15963, 60%) were more affected. The highest peak of malaria cases was reported during autumn season (September, October, and November) followed by spring season (March, April, and May).
Conclusions: Male and those age groups ≥ 15years were more affected than the others. The highest peak malaria prevalence was appeared from September to December (autumn season). Therefore, proper planning, implementation and monitor of malaria prevention and control activities should be strengthened at all levels.
Malaria prevalence, East Wollega, Western Ethiopia, Trends
API: Annual Parasitic Incidence; AR: Attack Rate; dhis2: District Health Information System Version Two; PF: Plasmodium falciparum; PK: Plasmodium knowlesi; PM: Plasmodium malariae; PMT: Performance Monitoring Team; PO: Plasmodium ovale; PV: Plasmodium vivax; RDT: Rapid Diagnostic Test; SNNP: Southern Nation, Nationalities and People; WHO: World Health Organization
Formerly Malaria is caused by infection of red blood cells with protozoan parasites of the genus plasmodium inoculated into the human host by a feeding female anopheline mosquito. The five human plasmodium species transmitted from person to person are plasmodium falciparum (PF), plasmodium vivax (PV), plasmodium ovale (PO)-two species, and plasmodium malariae (PM). Human infections with the monkey malaria parasite (zoonotic) plasmodium knowlesi (PK) are being reported from the forested regions of South-East Asia and particularly the Island of Borneo. The first symptoms of malaria are non-specific and similar to those of a minor systemic viral illness which includes headache, lassitude, fatigue, abdominal discomfort and muscle and joint aches, usually followed by fever, chills, perspiration, anorexia, vomiting and worsening malaise. Malaria is suspected clinically primarily on the basis of fever or a history of fever. All cases of suspected malaria should have a parasitological test (microscopy or rapid diagnostic test (RDT)) to confirm the diagnosis [1-2].
In 2018, an estimated 228 million cases of malaria occurred worldwide. Most of malaria cases during the same period were in World Health Organization (WHO) African Region (213 million or 93%), followed by the WHO South-East Asia Region with 3.4% of the cases and the WHO Eastern Mediterranean Region with 2.1%. Nineteen countries in sub-Saharan Africa, and India carried almost 85% of the global malaria burden. PF is the most prevalent malaria parasite in the WHO African Region, accounting for 99.7% of estimated malaria cases in 2018 [3]. The studies conducted in countries ranging from Pakistan to Kenya revealed a malaria prevalence of 6.8%,19.7%,33.6%,46.19%,61.5%,78.7%,a nd 36.5% respectively [4-10].
Two-third (68%) of Ethiopia’s land mass is favourable for malaria transmission and about 60% of Ethiopia’s population live in malarious areas, with malaria primarily associated with altitude (below 2,000 meters) and rainfall. The peak of malaria incidence follows the main rainfall season (July to September) each year. Malaria prevalence was 1.2 percent and 0.5 percent by rapid diagnostic test (RDT) and microscopy, respectively. PF was the species of malaria detected in 82% of positive cases according to RDT and 88% according to microscopy, while PV was the species in 8% of positive cases by RDT and 9% by microscopy, among all age groups [11].
Trends of malaria prevalence in Ethiopia from 2001-2011 revealed that confirmed malaria cases decreased from a mean of 78,325 during 2001-2005 to 30,780 cases in 2011. The slide positivity rate (SPR) was 24% in 2001 and 11% in 2011 [12]. PF and PV are the two dominant parasite species causing malaria in Ethiopia, with relative frequencies of about 60% and 40%, respectively. This proportion varies from place to place and from season to season [13]. Another similar study done in Ethiopia revealed that overall prevalence of malaria parasitaemia was 2.49% and 2.36% as detected using rapid diagnostic test (RDT) and microscopy, respectively. PF accounted for 62.63% of the infections. Infection rate was higher among males (2.7%). Overall, 126(18.75%) of the children were positive for total malaria antibodies with significant variations with altitude, age and sex; children ≥ 5 years (22.1%) and among males, 20.9% [14]. Besides, the study conducted in Ethiopia revealed that the combined, PF, PV, and mixed infections pooled prevalence estimates were 25.8%, 14.7%, 8.7%, and 1.2%, respectively. Based on agro ecological subgroup analysis, the highest malaria prevalence (37.6%) was obtained from studies conducted on mixed regions of low lands and midlands while the least (20.7%) was from low lands. In Ethiopia, malaria transmission is seasonal, variable, and coincides with the peak agricultural activities that greatly affected the country’s socio-economic development [15].
The study conducted in Amhara, Oromia and Southern Nations, Nationalities and Peoples’ (SNNPs) regions of Ethiopia showed that malaria blood slides were examined from 11,601 people. The overall prevalence of a positive blood slide (any species) was 4.1%. The highest prevalence of 5.4% was in SNNPR, followed by Amhara at 4.6%, with lowest prevalence of 0.9% in Oromia.
By species, 57% of infections were due to PF. The age-specific prevalence was 4.6% in children under five years; 4.2% in 5-14 year olds; 3.8% in 15-49 year olds and 4.4% in those aged 50 and over [16].
The study conducted in Jimma town, south western Ethiopia from July 2014 to June 2015 showed that a total of 1434 suspected malaria cases were examined from the health facilities and 428(29.85%) confirmed malaria cases were found. Among the confirmed cases, 327(76.4 %) were PV, 97(22.7 %) were PF, and four (0.9 %) were mixed infections of PV and PF. The annual malaria incidence rate was 1.7 cases per 1000 people at risk. Parasite prevalence in the community was less than 3%. Being male and traveling were the significant risk factors for PF malaria [17].
The study conducted in Oromia region, Ethiopia revealed that during 2001-2006, a total of 905,467 and 562,996 clinical and confirmed malaria cases, respectively, were reported with 80.2% clinical and 72.2% confirmed malaria cases seen at health centers. The study identified that 29.0% of outpatient visits to health facilities in certain administrative zones during high transmission years were for malaria Clinical and confirmed disease varied between zones; 5 of the 15 zones in Oromia (East Hararge, East Shoa, East Wellega, Jimma, West Hararge) reported >75% of the clinical cases seen at health facilities during 2001-2006 [18].
The study conducted in Serbo health center, Ethiopia showed that of the total 6863 smears, 3009 were found to be positive and contribute 43.8% of diagnostic yield. PF constituted the most predominant 64.6%, while PV confirmed with 34.9% cases. Among patients who underwent diagnostic testing and treatment for malaria, males 63.8% were more prone to have a positive malaria smear than females 36.2%. Chi-square statistical analysis shows that there was a statistically significant association found between male cases and number of positive blood smear. The most affected age group was 31-40 years 56.7% followed by 21-30 years age group (48.1%), and the least affected age group was 41-50 years, 39.9% [19].
The study conducted in Arjo Didhessa sugar cane plantation area, East Wollega, Western Ethiopia revealed that the overall malaria prevalence was 3.1%; Plasmodium vivax 8(57%) and Plasmodium falciparum 6(43%) were detected during the survey [20]. A similar study done in SibuSire district, Western Ethiopia revealed that 6036 (20.07%) microscopically confirmed malaria cases were reported in the health center and PF becoming a predominant species. The result of the study showed that malaria prevalence was higher in males than females. From the infected patients (53.6%) were male and (46.4%) were female but the difference was not statistically significant. The age group between 15-44 years was more affected, with a prevalence rate of (48.1%), followed by 5-14 years old and 1-4 years old with the prevalence rate (28.2%) and (15.4%), respectively. On the other hand, children below 1years old and above 64 years old were less affected with prevalence rate of 2.3% and 1%, respectively. But the difference was statistically not significant. PF and PV accounted for 66.1% and 30.5% of malaria cases, respectively and the mixed (both PF and PV) accounted 3.4% [21].
Malaria is a common and life-threatening disease in many tropical and subtropical areas [22]. In 2016, 91 countries reported a total of 216 million cases of malaria, an increase of 5 million cases over the previous year. In 2016, there were an estimated 445,000 deaths from malaria globally, compared to 446,000 estimated deaths in 2015. Most malaria cases in 2016 were in the WHO African Region (90%), followed by the WHO South-East Asia Region (3%) and the WHO Eastern Mediterranean Region (2%). Of the 91 countries reporting indigenous malaria cases in 2016, 15 countries-all in sub-Saharan Africa, except India-carried 80% of the global malaria burden. PF is the most prevalent malaria parasite in sub-Saharan Africa, accounting for 99% of estimated malaria cases in 2016 [23].
In 2018, there were an estimated 405,000 deaths from malaria globally. Children aged less than 5 years are the most vulnerable group affected by malaria. In 2018, they accounted for 67% (272,000) of all malaria deaths worldwide. The WHO African Region accounted for 94% of all malaria deaths in the same year. In 2018, about 11 million pregnancies in moderate and high transmission sub-Saharan African countries would have been exposed to malaria infection. Overall, about 24 million children were estimated to be infected with PF in 2018 in sub-Saharan Africa, and an estimated 1.8 million of them were likely to have severe anaemia. In 2018, an estimated US$ 2.7 billion was invested in malaria control and elimination efforts globally by governments of malaria endemic countries and international partners. Nearly three quarters of investments in 2018 were spent in the WHO African Region [3].
In 2016, an estimated US$ 2.7 billion was invested in malaria control and elimination efforts globally by governments of malaria endemic countries and international partners [23]. Despite being preventable and treatable, malaria continues to have a devastating impact on people’s health and livelihoods around the world. According to the latest available data, about 3.2 billion people were at risk of the disease in 97 countries, territories and areas in 2013, and an estimated 198 million cases occurred. In the same year, the disease killed about 584,000 people, mostly children aged less than 5 years in sub-Saharan Africa. In most countries where malaria is endemic, the disease disproportionately affects poor and disadvantaged people, who have limited access to health facilities and can barely afford the recommended treatment [1].
Malaria is a major public health problem in Ethiopia and has been consistently reported as one of the top three leading causes of morbidity and mortality. Ethiopia is also one of the most malaria epidemic-prone countries in Africa. Rates of morbidity and mortality increase dramatically (that is 3-5 fold) during epidemics [13]. In Ethiopia, transmission season is from September-December, April-May, (seasonal and unstable) coincides with major harvesting season; aggravate economic loss [24]. The peak of malaria illness incidence usually follows the main peak rainfall season (June to September) each year [25].
A time Series Analysis of Trends in Malaria Cases and Deaths at Hospitals from 2001-2011 done in Ethiopia revealed that among all ages, confirmed malaria cases in 2011 declined by 66% and slide positivity rate (SPR) by 37% compared to the level predicted by pre intervention trends (2001-2005). In children under 5 years of age, malaria admissions and deaths fell by 81% and 73% respectively. Optimal breakpoint of the trend lines occurred between January and June 2006, consistent with the timing of malaria interventions [12].
In the year 2016 there were an estimated 2,927,266 new malaria cases in Ethiopia. It caused an estimated 4,782 deaths with a crude death rate of 4.7/100,000 and Age-standardized death rate (ASDR) of 4.9/100,000 population. Malaria related mortality in Ethiopia have contributed for 2.8% of infectious and parasitic disease mortality and 0.7% of all deaths by the year 2016. Similarly, malaria mortality in Ethiopia has contributed for 1.2% of malaria related mortality in Africa and 1.07% of global malaria mortality. Mortality due to malaria was highest among males and under five children. The population at risk was increased by 16.75% between 2010 and 2016. Ethiopia still have high burden of malaria which accounts for 6% of global malaria cases and 12% of the global cases and deaths due to PV [26].
The study conducted in Chewaka District, Western Ethiopia showed that on average, each household comprised 2 malaria cases in the past one year period and the prevalence of malaria in the study setting was 32%. The mean annual cost of malaria illness to households was US$16, and most of this cost (78%) was contributed by the indirect costs. In every household, on average, patients and companions or caregivers lost 3.4 productive workdays due to malaria illness, respectively. Fourteen households out of 100 spent more than 5% of their annual income on malaria treatment and hence, they were prone to high economic burden or catastrophic costs. The mean direct cost of malaria illness was US$14.5 and most of the direct cost of malaria illness (55%) was comprised of medical costs [27].
The study conducted in Oromia and SNNPR regions of Ethiopia showed that the overall malaria parasite prevalence was 2.4%, but differed markedly between the two regions: Oromia, 0.9%; SNNPR, 5.4% [28]. Another similar study conducted in Oromia regional state on the distribution and magnitude of malaria showed that from a total of 190 woredas and 6,107 kebeles of the region, 172 (90.5%) woredas and 3,932 (64.4%) kebeles were found to be partially or completely prove to malaria, with about 65% of the total population residing in these areas. A total of 6,214,132 malaria cases were diagnosed and treated microscopically or clinically during 1995-2000 with an annual average of 1,242,826. Malaria cases accounted for 11.7% from the total number of outpatients registered at all health facilities during the period. The two most important causes of malaria during the period were P. falciparum and P. vivax, comprising of 51.5% and 32.3% of the case respectively. The disease affected all age groups of the population. All age groups were affected with relatively more cases of malaria in the age group 15 and above. Children under the age of five accounted for about 18% of the total population of Oromia and contributed about 16.7% of the total confirmed malaria cases reported in hospitals and health centers during the study period. There was an increasing trend in the proportion of confirmed malaria cases in under-five children from 15.7% in 1995/6 to 16.8% in 1996/7, 17.5% in 1997/8, 17.6% in 1998/9, except that it was 15.8% in 1999/2000 [29].
The study conducted in in East Shewa Zone of Oromia Regional State, Ethiopia revealed that of 810 suspected adult malaria patients who participated in the study, 204 (25%) had microscopically confirmed malaria parasites. The dominant Plasmodium species were PV (54%) and PF (45%), with mixed infection of both species in one patient [30]. Similar study done in Arsi Negelle health center, southern Ethiopia indicated that 11.45% microscopically confirmed malaria cases were reported with a fluctuating trend from a total of 22,025 malaria suspected patients gave blood film for malaria diagnosis in the past five years (2009-2013). With regards to sex disaggregation, 55% were males and 45% were females. With regard to plasmodium species, PV and PF accounted for 74% and 19.8% respectively. Mixed infection was reported as 6.2% of malaria prevalence throughout the five year period in the study area. Children in the age range of 0 to 5 years were the most affected by disease (22.8%), followed by 16 to 20 years age groups (17.8%). The highest peak of malaria cases was reported during spring seasons (32.3%) followed by summer seasons (31.8%), and the minimum (16.2%) malaria cases were observed during winter season. At species level, the highest number of slides positives of PF was recorded in spring and summer, PV peak were observed in spring followed by autumn while the highest cases of mixed infection observed in summer and minimum cases were recorded in winter seasons [31].
In East Wollega Zone public healthcare facilities there is lack of empirical evidences on the level of malaria prevalence and its associated factors. Therefore, the present study aimed to determine the trend of malaria prevalence in the last two years’ period from July 2018 to June 2020 in public health care facilities of East Wollega Zone, Western Ethiopia, 2020. The assessment and analysis of malaria morbidity trend in every malarious area helps to understand the dynamics of disease transmission. Such information is vital to develop evidence-based and area specific interventions. Hence, the aim of this study is to analyse the 2 years trends of malaria transmission in East Wollega Zone, Western Ethiopia.
Study setting and periods
The study was conducted in East Wollega zone, which is one of the 21 zones of Oromia region. Nekemte is its capital town, located at the distance of 328 km to the west of the capital city of Ethiopia., Addis Ababa. Administratively the zone is divided into 17 districts (locally termed “woredas”), 1 special town administration, 43 urban and 287 rural Kebeles (the smallest administrative unit in Ethiopia). All of 17 districts and 242 Kebeles are malaria endemic areas in the zone. The zone provides, health services for populations of more than 1, 593, 926. Also 3 governmental hospitals and 64 governmental health centers are serving the population of the zone. The mean annual temperature of the zone ranges from 0C 10.9-0C 33.9 and mean annual rainfall ranges from 1000-2400 millimeters, with peak rainy seasons usually observed during June through August (summer), locally termed as “Gana,” and September through November (autumn), locally termed as “Bira”. The Zone located within 8° 31.52 “-10° 19’44 “N latitude and 36° 07’51-37° 11’52“E longitude. It is composed of 3 agro-ecological zones: 4.91% highland (“Baddaa”), 53.17% midland temperate (“Bada daree”), and 41.92% lowland (“Gammoojjii”). In all public health care facilities malaria is tested by using rapid diagnostic testing (RDT) at health posts, and microscopy at both health centres and hospitals [32].
Study design
A retrospective study was carried out to assess the two year (July 2018 to June 2020) trend analysis of suspected malaria cases based on dhis2 data-base reports.
Source of information
The source of information was the dhis2 data-base of East Wollega Zone. Monthly report of malaria cases of 17 districts was documented in the dhis2 data base. All districts were included in the study. All 17 district’s Public health facilities (hospitals and health centers) that provide diagnosis and treatments of malaria cases were included in the study. All 17 district’s Public health facilities uses similar monthly malaria report formats and the variables captured was similar regardless of districts or types health facilities.
Inclusion and exclusion criteria
Since it was retrospectively study, a total of two year period (July 2018 to June 2020) suspected and confirmed individuals for malaria cases reports were included in the study, and all incomplete data were excluded.
Data collection tools and techniques
Secondary data of monthly reported suspected and confirmed malaria cases were extracted from the dhis2 data-base of East Wollega Zonal health office by using a standardized checklist and questionnaire, which were adapted from Federal ministry of Health of Ethiopia National Malaria guideline, 2018, and previous related literatures. The contents of questionnaire include: Districts name, total population, sex, age group, seasonal patterns, reporting date, total blood Film (BF) examined by Rapid Diagnostic Test (RDT) and/or microscope, total confirmed positive malaria cases, and malaria species. A monthly two-year malaria data (from July, 2018 to June, 2020) was extracted to districts and zone through filtering of important variables from dhis2 database from September to November, 2020 and filtered data were used for analysis. All malaria cases reported from July, 2018 to June, 2020 were carefully reviewed by using questionnaire and analyzed.
Data processing and analysis
Data were first entered, processed and analyzed into Microsoft Excel 2010. A descriptive analysis, using mean and percentage was calculated. Different graphs and tables were used to present trends of malaria cases and total population.
Data quality assurance
One day training was given for each data collectors and supervisors. Data collectors were supervised by Zonal malaria expert. Regular supervision was given during data collection. Collected data was checked by supervisors before sent to the data entry on daily basis. To ensure the quality of data, selected district’s malaria report was evaluated for completeness and all the needed information was checked. To avoid bias, district’s monthly malaria cases report with redundant, incomplete or missing information was omitted.
Operational definition
Confirmed malaria: Suspected malaria case in which malaria parasites have been demonstrated in a patient’s blood by microscopy or a rapid diagnostic test.
Suspected malaria case: Clinical diagnosis of malaria is made in a patient who has fever or history of fever in the last 48 hour and lives in malaria-endemic areas or has a history of travel within the last 30 days to malaria endemic areas.
Slide positivity rate (SPR): Proportion of RDT and microscopy slides found positive among the slides examined.
Population at risk: Population living in a geographical area in which locally acquired malaria cases occurred in the current and/ or previous years.
Annual parasite incidence (API): Total number of positive slides for malaria parasite in a year × 1000 per total population at risk.
Malaria prevalence: Total number of confirmed cases during a specified period of time x 100 per total population at risk during the same period.
Attack rate: Total number of confirmed cases during a specific period of time x 100 per total population during a specific period of time.
Ethics considerations
Before data collection; official letters was gained from East Wollega Zonal Health Office, Oromia Regional State, Western Ethiopia. The purposes and the importance of the study were stated as the unique objective of the study for the zone to serve as base line information for malaria case team, and for different stake holders. East Wollega Zone disease prevention and control is the owner of this two years data and it direct stakeholder or programmer for malaria prevention and intervention measures. Confidentiality was assured at all levels of the study using password protected computer and removing patient identifiers.
Annual trends of malaria burden
Since July 2018 to June 2020, a total of 125,917 suspected malaria cases were examined from all districts of East Wollega Zone. The total number of confirmed malaria cases was 26,679 making an overall slide positivity rate (SPR) of 21.20%. During the specified period the overall attack rate (AR) was 16/1,000 population with zero fatality rate. A confirmed malaria case was reported in all districts found in the Zone. However, high malaria AR was reported from Sassiga, Diga, Boneya Boshe, Guto Gida, Leka Dulecha and Jima Arjo districts (Table 1).
| Name of the district | Total Population | Total blood film Examined | Confirmed malaria cases | AR in % |
|---|---|---|---|---|
| Boneya Boshe | 68,470 | 11682 | Â Â Â Â Â Â Â 1,970 | Â Â Â Â Â Â Â Â Â Â Â 2.9 |
| Diga | 96,419 | 12158 | Â Â Â Â Â Â Â 3,081 | Â Â Â Â Â Â Â Â Â Â Â 3.2 |
| Ebantu | 53,158 | 2235 | Â Â Â Â Â Â Â Â Â Â 332 | Â Â Â Â Â Â Â Â Â Â Â 0.6 |
| Gida Ayana | 151,290 | 10442 | Â Â Â Â Â Â Â 1,563 | Â Â Â Â Â Â Â Â Â Â Â 1.0 |
| Gobu Seyo | 78,980 | 4864 | Â Â Â Â Â Â Â Â Â Â 782 | Â Â Â Â Â Â Â Â Â Â Â 1.0 |
| Gudeya Bila | 59,203 | 6386 | Â Â Â Â Â Â Â Â Â Â 370 | Â Â Â Â Â Â Â Â Â Â Â 0.6 |
| Guto Gida | 78,906 | 9968 | Â Â Â Â Â Â Â 2,217 | Â Â Â Â Â Â Â Â Â Â Â 2.8 |
| Haro Limu | 115,493 | 6717 | Â Â Â Â Â Â Â 1,630 | Â Â Â Â Â Â Â Â Â Â Â 1.4 |
| Jima Arjo | 75,237 | 5824 | Â Â Â Â Â Â Â 1,365 | Â Â Â Â Â Â Â Â Â Â Â 1.8 |
| Kiramu | 124,427 | 7298 | Â Â Â Â Â Â Â 2,073 | Â Â Â Â Â Â Â Â Â Â Â 1.7 |
| Leka Dulecha | 103,023 | 6833 | Â Â Â Â Â Â Â 2,089 | Â Â Â Â Â Â Â Â Â Â Â 2.0 |
| Limu | 103,653 | 4047 | Â Â Â Â Â Â Â Â Â Â 867 | Â Â Â Â Â Â Â Â Â Â Â 0.8 |
| Nunu Kumba | 92,889 | 7903 | Â Â Â Â Â Â Â Â Â Â 764 | Â Â Â Â Â Â Â Â Â Â Â 0.8 |
| Sasiga | 115,086 | 12904 | Â Â Â Â Â Â Â 4,639 | Â Â Â Â Â Â Â Â Â Â Â 4.0 |
| Sibu Sire | 147,200 | 7666 | Â Â Â Â Â Â Â 1,540 | Â Â Â Â Â Â Â Â Â Â Â 1.0 |
| Wama Hagalo | 69,565 | 6782 | Â Â Â Â Â Â Â Â Â Â 601 | Â Â Â Â Â Â Â Â Â Â Â 0.9 |
| Wayu Tuka | 89,508 | 2208 | Â Â Â Â Â Â Â Â Â Â 796 | Â Â Â Â Â Â Â Â Â Â Â 0.9 |
| Zonal total | 1,622,507 | Â Â 125,917 | Â Â Â Â 26,679 | Â Â Â Â Â Â Â Â Â Â Â 1.6 |
Table 1: Total malaria cases and attack rate in each district of East Wollega Zone over the study period (July 2018 to June 2020), Nekemte town, Western Ethiopia, 2020.
Regarding the identified plasmodium species, both species of plasmodium were reported in each year with PF being the predominant species in the study area. In the study area, PF and PV accounted for 80% and 20% of malaria morbidity, respectively. There is no mixed infection reported during the study period (July 2018 to June 2020).
Prevalence of malaria cases in relation to sex and age
Based on health management information system(HMIS) classification, malaria was reported in all age groups in the study area but the age group of ≥ 15years were more affected, with a prevalence rate of 15,963 (60%), followed by 5-14 years olds and <5years olds with the prevalence rate 7,170 (27%) and 3,546 (13%), respectively. It is also indicated that males were more affected than females. The infection rates among males were 14,802 (55.5%) and females were 11,877 (44.5%) with male to female ratio of 1.2 (Table 2).
| Variables | Category | Total confirmed malaria cases from July 2018-June 2020. | |
|---|---|---|---|
| Number | Percentage (%) | ||
| Age group | <5years | 3546 | 13 |
| 5-14years | 7170 | 27 | |
| =15years | 15963 | 60 | |
| Total | 26679 | 100 | |
| Sex | Male | 14802 | 55.50 |
| Female | 11877 | 44.50 | |
| Total | 26679 | 100 | |
Table 2: Total malaria cases by sex and age at public healthcare facilities of East Wollega Zone from July 2018-June 2020, Nekemte town, Western Ethiopia, 2020.
The annual parasitic incidence (API) cases showed almost similar trend during the last 2 years period, from July 2018 to June 2020. It was 8.54 cases per 1000 people at risk in the year 2018/2019 and 8.23 cases per 1000 people at risk in the year 2019/2020. This is classified as moderate malaria strata (Table 3).
| Year | Population at risk | Total number of confirmed malaria cases | Annual parasitic incidence per 1000 population(API/1000) |
|---|---|---|---|
| 2018/2019 | 1578161 | 13472 | 8.54 |
| 2019/2020 | 1604216 | 13207 | 8.23 |
Table 3: Annual parasite incidence in public healthcare facilities of East Wollega Zone in the last two years period (July 2018 to June 2020), Nekemte town, Western Ethiopia, 2020.
With regards to each district in the last two years period (July 2018- June 2020), malaria case was reported in all age groups in the area but those age group ranging ≥ 15 years, constituted majority of cases and the minimum malaria cases were reported in the age groups of <5 years (Table 4).
| District name | Total number of slides or RDT performed for malaria diagnosis | Total number of slides or RDT positive for malaria cases | Malaria cases by age category | |||||
|---|---|---|---|---|---|---|---|---|
| <5years | 5-14 years | =15years | ||||||
| N | % | N | % | N | % | |||
Table 4: The SPR among different age groups in the last two years (July 2018 to June 2020) at each district of East Wollega Zone, Nekemte town, Western Ethiopia, 2020.
Seasonal distribution of malaria
Despite the apparent fluctuation of malaria trends in the study area, malaria cases occurred in almost every month and season of the year. The highest peak of malaria cases in almost all months of the years was observed during October, November, December and June, and the minimum malaria cases were observed during February, March and April (Figure 1 and 2). The SPR shows increment in some months (June-September) in last one year (July 2019 to June 2020) than the previous year (July 2018 to June 2019) (Figure 2).
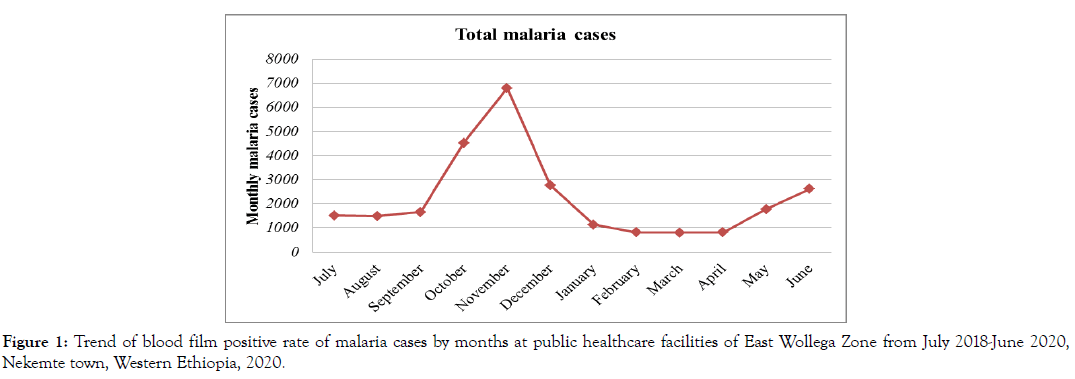
Figure 1: Trend of blood film positive rate of malaria cases by months at public healthcare facilities of East Wollega Zone from July 2018-June 2020, Nekemte town, Western Ethiopia, 2020.
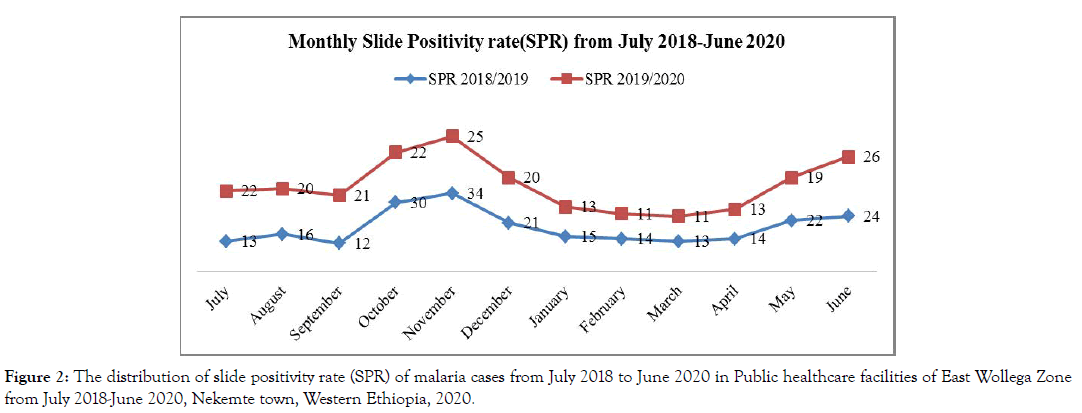
Figure 2: The distribution of slide positivity rate (SPR) of malaria cases from July 2018 to June 2020 in Public healthcare facilities of East Wollega Zone from July 2018-June 2020, Nekemte town, Western Ethiopia, 2020.
The highest peak of malaria cases were observed during the Autumn (September, October, November) sometimes called harvest season, followed by Spring (March, April, May) and the least malaria cases during Winter season (December, January, February) (Figure 3).
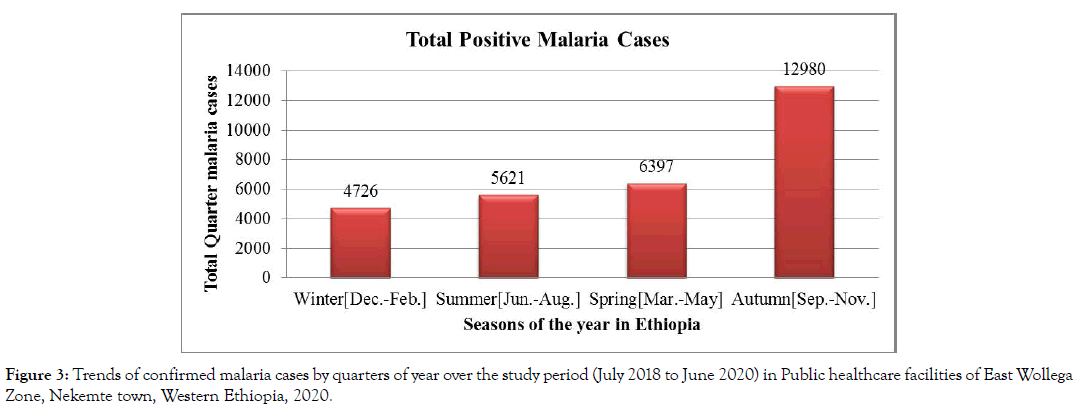
Figure 3: Trends of confirmed malaria cases by quarters of year over the study period (July 2018 to June 2020) in Public healthcare facilities of East Wollega Zone, Nekemte town, Western Ethiopia, 2020.
The present study attempted to assess the trend of malaria prevalence in public healthcare facilities of East Wollega Zone from dhis2 data extraction, Western Ethiopia.
An overall malaria prevalence of 21.2% was recorded in the present two-year retrospective study in public healthcare facilities of East Wollega Zone. This result was markedly lower than many studies in the country and abroad [6-10,17-19,27,33-39]. On the other hand, it was higher than the study findings conducted in different areas [4,5,16,20,28,31,40-46]. The current assessment result is consistent with the previous retrospective studies conducted in Boricha district, southern Ethiopia, with 21.8% of prevalence, Dembia district, North West Ethiopia with 21.8%, and Sibusire district, Western Ethiopia with 20.07% [21,47,48]. The possible contributing factors for the observed variation could be the type of study design used, time variations of the studies, duration of study period (only the last two years malaria cases is collected in this assessment), variations in geographical locations, climatological differences, altitude variation, malaria diagnosis technique variation, skill of the laboratory personnel to detect and identify malaria parasites, expansion of development projects like dams or irrigation, differences in population awareness about malaria transmission and intervention practices, and health seeking behaviour, and other factors that affect malaria case occurrences in different study areas.
Concerning the distribution and magnitude of malaria burden, this finding showed that all districts in the Zone reported malaria cases in the specified period. Districts like Sassiga, Diga, Boneya Boshe, Guto Gida, Leka Dulecha and Jima Arjo have reported high malaria AR within the study period. The possible explanation for this malaria cases prevalence in some districts were huge stagnant water is available in this districts which is a potential breading site for mosquitoes, weak utilization of long lasting integrated bed nets, weak community based surveillance system, and weak early case detection and management. Such result is comparable with other study conducted in other area [29].
With regard to plasmodium species, this assessment revealed that the burden of malaria was high in the study area where the most deadly species, PF, accounted for 80% of malaria morbidity. PV only accounted for 20% of malaria morbidity and no mixed infections is reported in the study area. This finding is in agreement with the malaria parasite distribution in Ethiopia which indicates that PF and PV are the two predominant malaria parasites, distributed all over the country and accounting for 60% and 40% of malaria cases, respectively [13]. This finding was comparable with the results of studies conducted in different areas of Ethiopia and other countries [9-21,29,33-39,45,46,48-52]. This finding was in contrast to the studies result conducted in Jimma town, Arjo Didessa, East Shoa, Arsi Negelle, Halaba special district, and Wolkite healthcenter which reported PV as the dominant plasmodium species in the area [17,20,30,31,41,43]. The possible reason for this trend shift from PF to PV might be due to the public health importance of PV that is frequently overlooked and left in the shadow of the enormous problem caused by PF, the prevention and control activities of malaria as guided by the National Strategic Plan (2006-2010) mainly focus on PF because it is assumed to be more prevalent and fatal malaria in the country Ethiopia, climate variability, inconsistency malaria preventing methods in the areas, and gap of programme performance [33]. Moreover, PV relapses from dormant liver stages at varying time intervals after the initial infection.
Regarding the age groups, ≥ 15 years (60%) were highly affected age groups followed by 5-14(27%) years old and under five children (13%). This finding is in agreement with other studies conducted in various areas, which reported higher malaria prevalence in the age groups of ≥ 15 years [21-39,41-53]. In contrast, higher prevalence malaria was reported in children less than five years of age and between 5-14years old elsewhere in the country and other areas [8-10,31-37,54-56]. The observed lower prevalence of malaria in children under 5 years of age and between 5-14 years old might be because of their less likely exposure to infected mosquito bite due to good awareness and practices of their parents or care takers on malaria control and prevention activities. The possible explanation why the productive age group, ≥ 15 years old highly affected by malaria cases might be due to the fact that in all districts of the Zone agriculture is the main occupation and the area is malarious so staying outside the home makes them more exposed to anopheles mosquito bites, which can transmit malaria parasites. Besides, most of those in age group 5-14 were students in rural areas of the surrounding because they had relocate for education purpose and as such get the infection since they were from nonmalaria area previously.
The study also revealed higher positivity rate of malaria among males (55.5%) than females (44.5%) with male to female ratio of 1.2. This finding is comparable with other studies conducted in various areas, which reported higher malaria prevalence in males than females [14-21,31-39,43-48,51-53]. In contrary, a study conducted in Pakistan, Bunkure of Nigeria, and Halaba special district of Ethiopia reported a lower prevalence of malaria in males than females [4,9,41]. Another studies conducted in ten Asian countries and Wolaita Zone, Ethiopia reported that the distribution of malaria cases among male and female is nearly equal [37,54]. The possible contributing factors for this difference may be due to the study area, relative differences with regards to gender roles, demographic variations (that is large number of females in some areas), temporal changes might be avoiding gender differences, and distribution of malaria risk is heterogeneous or other factors. The possible explanation why such higher malaria prevalence in males than females is considered to be attributed to the fact that males are usually spending most of their time in outdoor activities (like agricultural activities in the fields) especially during evenings when the peak biting activity of the infective mosquito is observed and put them at greater risk of contracting the disease. Moreover, this could be due to male is more responsible to control the environmental activities than female, long traveling habit to other sites and in most Ethiopian communities’ culture including the study area, females are restricted to home activities, like taking care of children and usually spend their time indoors.
This assessment reported the annual parasitic incidence (API) of 8.54 cases per 1000 people at risk in the year 2018/2019 and 8.23 cases per 1000 people at risk in the year 2019/2020. With regard to malaria strata, this is classified under moderate strata according to updated national malaria guideline (that is ≥ 5 and <100 is classified under moderate malaria strata) in the last two years in the study area. Based on this the proposed malaria intervention were distribution of long lasting integrated bed net, larval control where applicable, case management, surveillance, information, education and communication (IEC) and behavioural change communication (BCC). This result is higher than the study conducted in Jimma town, south western Ethiopia which was reported the API of 1.7 cases per 1000 people at risk [17].
In Ethiopia, malaria transmission coincides with the peak agricultural activities that greatly affected the country’s socioeconomic development [15]. This assessment revealed that malaria case occurs in all months and years in this study area. In this assessment the prevalence of malaria shows some increment in some months(June-September) in the year 2019/2020 than 2018/2019,because in the year 2019/2020 the number of suspected malaria cases examined (72,180) is higher than that of 2018/2019(55,270), long-rainy seasons which is favourable condition for mosquito density increment and increases malaria transmission in 2019/2020, illegal settlements in some districts like Diga (Sholo kebele and gote 17),and Sassiga (Bareda and Balo kebele),and poor data quality. The highest peak malaria case was observed during in October and November months (autumn season), December month (winter season) and June month (summer season) and lower during February, March and April months. This is in agreement with many studies conducted in different areas [4,47,48].
Seasonality and year played a role in the transmission of malaria in the study area. The highest peak of malaria cases in almost all year groups was observed during Autumn season (September, October and November) or sometimes called harvest season followed by Spring season (March, April and May), and the least malaria cases were reported in winter season (December, January, and February). This peak malaria season in the study area was similar with many studies done at different areas [24,25,31-39,40-48,51,52]. In contrary, the studies revealed that the peak malaria cases occurred during June-July, least in Autumn, and Spring seasons [8,35,48,51,53,48]. The possible explanation for this difference might be due to climatic variables, ecologic and environmental factors, vector characteristics, and rainfalls variations. This high prevalence of malaria cases occurs in autumn (September- November) is the major transmission of malaria follows the June to September heavy rainfalls which might be related to the formation of stagnant water after the heavy rainy season (summer), favourable temperature, and high vegetation density for mosquito breeding. The second peak malaria case was observed on spring season (March to May) which is the minor transmission season is following the February to March rains in the study area.
Strength of the study
• The strength of this study lies in the fact that malaria data were extracted from dhis2 data base by trained health information technicians (HIT), and malaria experts in the Zone.
• Besides, this study has also investigated the factors associated with the trends of malaria prevalence by collecting comprehensive information from Zonal level malaria expert.
Limitation of the study
• The data was collected from health facilities only and this might underestimate the actual burden of malaria in the community in the study area.
• This study was only limited to quantitative aspect.
• Moreover, the trends of malaria mortality were not reported in this study. Therefore, interpretation of the findings should be with the precaution.
Conclusion
Bacterial vaginosis is a very common polymicrobial condition that is sexually active and particularly of reproductive age. It is associated with many risk factors including race, geographic location, low socioeconomic level, age, number of pregnancies, alcohol consumption, frequency of sexual intercourse, and level of intimate hygiene.
The prevalence of bacterial vaginosis in sexually active women living in the city of Franceville and its surroundings is found to be quite high (64.59%) based on the Gram stain used to establish the Nugent score, reference. Based on socio-demographic survey sheet providing information on the participants' clinics, it appeared that Amsel's criteria were much less sensitive for the diagnosis of this pathology since more than half of the positive cases in this study were asymptomatic.
Finally, almost half of the pregnant women included in this study were suffering from bacterial vaginosis, but the small number recorded would warrant further investigation on a large panel of pregnant women to be able to conclude on the likely impact of this dysbiosis on the future of pregnancy and therefore on the fertility rate under our skies.
A high prevalence of malaria was observed during the study period in the Zone. In this present assessment the overall trends of total confirmed malaria case were minor increment in the last one year (July 2019 to June 2020) at some months. The result of this assessment revealed that malaria is a serious health burden in the area, in which PF is the predominant malaria parasite. Male and those age groups equals and above fifteen years (≥15years) were more affected by malaria in this study area. The malaria prevalence appeared to follow a fluctuating trend, where it peaks from September to December. The highest peak of malaria cases in almost all year groups was observed during the Autumn seasons (September, October, and November) followed by Spring seasons(March, April, and May).
Recommendation
This study had shed light to the trends of malaria cases in the last two years (July 2018 to June 2020) in public healthcare facilities of East Wollega Zone, Western Ethiopia. Based on this finding, we give the following recommendation for concerned bodies.
• Primary healthcare units’ staffs (District hospitals; health centers; and health posts staffs) should give more attention to awareness creation for the local community in the practice and proper implementation of the malaria control activities, and promote their health seeking behaviours.
• District health office and primary healthcare units’ performance monitoring team (PMT) is recommended to monitor and evaluate malaria cases data management system.
• Zonal and District health office should give due attention for environmental management, malaria vector control, early diagnosis and treatment of malaria cases, and strengthen the surveillance system at each level.
• Oromia Regional Health Bureau, Zonal and District health office is also recommended to conduct continuous supervision of malaria prevention and control activities to deduce methods to improve healthcare service delivered by public healthcare facilities according to national standards.
• A regional laboratory was recommended to monitor the quality of test and provide on job training for laboratory personnel at facility level.
• We highly recommended others researchers to conduct a detailed and extensive study by adding another explanatory variables and qualitative aspects to get the clear picture of the whole situation of the malaria cases in the study area.
Ethics approval and consent to participate
Before data collection; official letters was gained from East Wollega Zonal Health Office, Oromia Regional State, and Western Ethiopia. The purposes and the importance of the study were stated as the unique objective of the study for the zone to serve as base line information for malaria case team, and for different stake holders. East Wollega Zone disease prevention and control is the owner of this two years data and it direct stakeholder or programmer for malaria prevention and intervention measures. Confidentiality was assured at all levels of the study using password protected computer and removing patient identifiers.
Availability of data and materials
The finding of this study is generated from the data collected and analyzed based on stated methods and materials. The original data supporting this finding are available from the corresponding author on reasonable request.
Babure ZK participated in the design of the study, performed the data collection and the statistical analysis and served as the corresponding author of the manuscript. Ahmed YM, Likasa ST, Jiru FA, Weldemarium TD, and Fite MB supervised the study, ensured quality of the data, assisted in the analysis and interpretation of the data. All authors read and approved the manuscript.
We are grateful to East Wollega Zonal Health office disease prevention and control case team staff, for their unfailing support, and guidance during the whole activities of this thesis. We also indebted for monitoring and evaluation case team staff of East Wollega Zonal health office for their valuable effort and participation in this study. Finally our thanks go to disease prevention and control case team staffs of all district health office in the Zone for their suggestion and ideas during the activities of this thesis.
Citation: Babure ZK, Ahmed YM, Likasa ST, Jiru FA, Weldemarium TD, Fite MB (2021) Trend Analysis of Malaria Prevalence in East Wollega Zone, Oromia Regional State, Western Ethiopia, 2020: A Retrospective Study. J Women's Health Care 10:515. doi:10.35248/2167-0420.21.10.515.
Received: 11-Jan-2021 Accepted: 08-Feb-2021 Published: 15-Feb-2021 , DOI: 10.35248/2167-0420.21.10.515
Copyright: © 2021 Babure ZK, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.