
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2023)Volume 6, Issue 1
Extensive vascularity and large amounts of collagen and elastin in the lung tissue makes the lung parenchyma vulnerable to diabetes. However, there are few studies on the pathophysiological effects of diabetes on lung tissue. Therefore, in this study, we investigated the impacts of Type 2 Diabetes (T2D) on lung tissue pathology and the expression of miRNA-155 and miRNA-133a in the lung tissue of male rats. In this study, 20 male rats were divided into a control group and a diabetic group. The diabetic group received a high-fat diet for four weeks. After the fourth week, the rats were injected with a single dose of Streptozotocin (STZ). Blood glucose and Glucose Tolerance Tests (GTT) were measured four days after the STZ injection; immediately after testing, rats were sacrificed, and lung tissue was collected to measure microRNA (miRNA) and examine tissue changes. When lung tissue sections from diabetic rats were examined, the normal structure of alveoli, alveolar sacs, and bronchioles was disrupted. The extensive alveolar collapse was the leading cause of lung tissue structure disruption, and the accumulation of inflammatory cells and exudate secretions resulted in an interstitial pneumonia-like appearance. The expression of miRNA-155 was increased, and the expression of miRNA-133a was decreased in the lungs of diabetic rats compared with control rats. We found significant changes in the lung tissue of diabetic rats. These miRNAs can be used as diagnostic biomarkers for lung injury in diabetic patients. In addition, the changes in these miRNAs may provide therapeutic strategies.
Animal models; miRNA-133a; miRNA-155; Pulmonary disease; Type 2 diabetes
Diabetes mellitus refers to several metabolic disorders, all characterized by chronic hyperglycemia. In this disease, the ability to produce the hormone insulin is lost (type 1), or the body's cells become resistant to insulin (Type 2 Diabetes (T2D)). When blood glucose levels increase and affect metabolism, the complications of the disease become apparent [1,2]. Cardiovascular, renal, and ocular complications, nervous system problems, peripheral organ disorders due to nerve damage, and cardiac and ischemic strokes are complications of diabetes in the body [3].
Lung tissue is highly susceptible to damage and microvascular complications in diabetes because of its large vascular extent and abundant connective tissue [4]. Decreased lung function in people with diabetes is directly related to blood glucose levels, duration of diabetes, and obesity [5]. Changes in the lung tissue of patients with diabetes include an increase in the thickness of the basement membrane, which is due to an inflammatory process in the lungs of a patient with diabetes, an infiltration and accumulation of neutrophils and an increase in alveolar wall thickness, and fibrosis toward infiltration of inflammatory cells [6]. Previous studies have shown pathological changes in the lung tissue of diabetic patients. However, the molecular and pathophysiological mechanisms of the effects of diabetes on lung tissue have not yet been elucidated, and much remains to be studied [5-7].
MicroRNA(miRNA)s are small, uncoded, protected RNAs 18-23 nucleotides in length that control gene expression after transcription by inhibiting translation or initiating degradation of mRNAs [8]. It has also been shown that each miRNA has hundreds of target genes. Thus, even if the expression of a miRNA is altered, it significantly affects the regulation of target genes [9]. Their expression may be a marker of tissue health or disease. These miRNAs can be used as biomarkers for diagnosis and prognosis [10]. The involvement of many miRNAs in diabetic complications such as retinopathy, nephropathy, neuropathy, and cardiovascular and pulmonary complications has been demonstrated [8,11]. The expression level of miRNA-133a is decreased in asthma [12]. In asthmatic mouse models, increasing the expression of this miRNA decreases airway remodeling (by affecting airway smooth muscle) [13]. Expression of miRNA-133a is decreased in the bronchial smooth muscle of asthma patients, which increases Ras homolog family member A (RhoA) levels and improves bronchial responsiveness and sensitivity [14]. miRNA-133a suppresses myofibroblast development and progression of pulmonary fibrosis by inhibiting TGF-1 in Human Lung Fibroblasts (HFL) [14]. The results of a study showed that miRNA-133a suppressed the production of traditional myofibroblast differentiation markers such as Smooth Muscle Actin (SMA), Connective Tissue Growth Factor (CTGF), and collagens in response to TGF-1 stimulation [15].
The level of miRNA-155 in the serum of patients with T2D is low [16], suggesting that miRNA-155 controls blood glucose levels in patients with diabetes. Genetically modified mice expressing higher levels of miRNA-155 were more likely to have hypoglycemia and increased insulin sensitivity [17,18]. In addition, the level of miRNA-155 in foot ulcers of study participants in the diabetes group is higher than usual, suppressing FGF7, which is effective in wound healing and wound epithelialization. Inhibition of miRNA-155 significantly increased the rate of wound healing [16]. In addition, studies of miRNA inhibition in preclinical models of asthma, Cystic Fibrosis (CF), and Idiopathic Pulmonary Fibrosis (IPF) have shown good therapeutic targets [19-21]. The aim of this study was to investigate the effects of T2D on lung tissue and changes in the expression of miRNA-155 and miRNA-133a in the lung tissue of rats. miRNA-155 and miRNA-133a can be considered diagnostic biomarkers of lung damage in diabetic patients.
Animals studied
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences under number 1398/344. For this study, 20 male Wistar rats (6-8 weeks old, 180-220 g) were brought to the Animal Care Center of Tabriz University of Medical Sciences after purchase. The study protocol was prepared following NIH guidelines for the care and use of animals and approved by the Ethics Committee for the Use of Animals in Research at Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1398.344). Animals were housed in standard cages in a 12:12 dark-light cycle at temperatures between 18 and 22°C and had free access to water and food. Ten days after transfection (to relieve stress and acclimate to the new environment), the rats were tested for diabetes. However, none of the rats were diabetic. Then rats were randomly divided into two groups: control (10 rats with a standard diet) and diabetic (10 rats with diabetes induction). Glucose above 250 mg/dl was considered diabetic. The study site was the Applied-Drug Research Center of Tabriz University of Medical Sciences.
Induction of diabetes and approval of diabetic rats
A high-fat diet acclimated T2D (wheat flour 10%, sucrose 20%, fat 31%, casein 25%, vitamin and mineral mixture 6%, methionine 0.3%, yeasts 0.1% and sodium chloride 0.1%) for four weeks, followed by an intraperitoneal injection of a single dose of Streptozotocin (STZ) (35 mg/kg body weight) [22,23]. To dissolve STZ, 0.1 M cold citrate buffer with a pH of 4.5 was used. Rats were randomly divided into two groups: In the control and diabetic groups, in the control group, the animals were fed the same standard diet for four weeks, and in the diabetic group, the animals were fed for four weeks. They were fed a high-fat, high-protein diet plus one dose of STZ (end of week 4) and were at risk for T2D. Four days after STZ injection, T2D developed in rats. A glucometer was used to diagnose and confirm diabetes. In this way, with a small injury by Lancet in the animal's tail, a drop of blood was placed on the glucometer tape. The glucometer then read the tape, and blood sugar above 250 mg/dl was considered an indicator of diabetes. Rats in the diabetic and control groups fasted for 12 hours. A GTT was then performed by administering 1 g/kg glucose to the diabetic and control rats. Blood glucose levels were measured after 0, 30 min, 60 min, 90 min, and 120 min using a rat glucose meter. GTT is a laboratory test to check the transfer of the amount of glucose used to body tissues such as muscle and fat. This test is often used to diagnose diabetes. All manipulations were held in the morning.
Preparation of lung tissue for analysis of tissue sections and expression of miRNAs
During the fifth week after the end of the test, lung tissue was collected for the measurement of miRNAs after animals were anesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg) to assess tissue changes. Lung tissue was washed with cold, sterile normal saline. Then, one lung was frozen to examine gene expression, and the other was fixed in a 10% formalin solution to examine tissue changes. The hematoxylin-eosin (H&E) method was used to evaluate the extent of lung injury. The histopathological examination included tissue changes such as leukocyte infiltration, perivascular edema, inflammation, and pulmonary fibrosis. Histopathological changes were examined using a light microscope (Olympus CH30 made in Japan) and photographed with an Olympus DP12 camera, U-TVO.5XC-2 Japan.
miRNAs extraction, cDNA synthesis and real-time polymerase chain reaction
Real-time Polymerase Chain Reaction (PCR) was used to determine the transcription of miRNA-155 and miRNA-133a. For this purpose, lung tissue was rapidly sectioned, and RNA content was extracted using TRIzol (Roche, Germany) according to the manufacturer's instructions. A Picodrop 1000 spectrophotometer (Thermo Scientific, USA) was used to determine RNA concentration and integrity. cDNA Synthesis Kit was used to generate cDNA (TaKaRa). Real-time PCR was performed on a Rotor-Gene 6000 instrument using a cocktail of cDNA sample (1L), SYBR Green Master Mix (5L; TAKARA), DEPC water (3.7L), and primers (0.3L) (Corbett, Australia). The sequences of the primers used are listed in Table 1. The PCR products were standardized to U6.
| Gene name | Primer sequence | Reference |
|---|---|---|
| miRNA-133a | Forward: 5'-GTGCATTTGGTCCCCTTCA-3' | |
| Universal Reverse: 5'-CGGGCTGTCAGTTTGTCA-3' | 24 | |
| Stem loop: 5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAGCTC-3' | ||
| miRNA-155 | Forward: CGGCGCTTAATGCTAATCGTGATAG | |
| Universal Reverse: GTGCAGGGTCCGAGGT | 11 | |
| Stem loop: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACCCCT | ||
| U6-6p | Forward: GCTTCGGCAGCACATATACTAAAAT | |
| Reverse: CGCTTCACGAATTTGCGTGTCAT | 11 | |
| Stem loop: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAATAT |
Table 1: The PCR products standardized to U6.
Statistical analysis
The student’s t-test was used to examine and analyze the data from the control and diabetic groups. The analysis was performed using SPSS 19 software. The student t-test was used to analyze all quantitative data presented as mean ± SEM. In this study, a value of P<0.05 was considered significant.
GTT confirms type 2 diabetes
Before GTT measurement, the weights of diabetic rats (230-250 g) and control rats (200-220 g) were measured. In addition, the blood glucose levels of the case and control groups were checked (Figure 1A) (P<0/001). GTT measurements showed that glucose tolerance was impaired in the diabetic group compared to the control group (Figure 1B) (P<0/001).
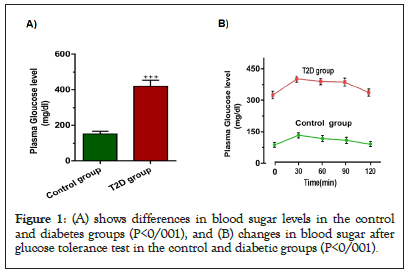
Figure 1: (A) shows differences in blood sugar levels in the control and diabetes groups (P<0/001), and (B) changes in blood sugar after glucose tolerance test in the control and diabetic groups (P<0/001).
The effect of diabetes on lung tissue
The lungs of both diabetic and control groups were examined by light microscopy with hematoxylin and eosin staining. The lung tissue sections of the rats in the control group showed normal tissue structure. Normal alveoli with the septum between the thin alveoli and alveolar sacs were visible. The alveoli walls were lined with type 1 and type 2 pneumocytes, each with its characteristics (Figure 2A).
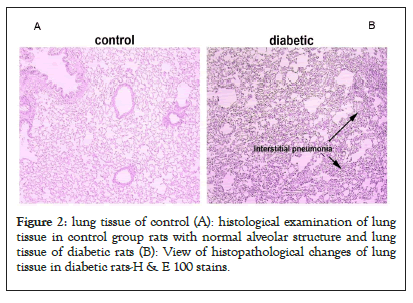
Figure 2: lung tissue of control (A): histological examination of lung tissue in control group rats with normal alveolar structure and lung tissue of diabetic rats (B): View of histopathological changes of lung tissue in diabetic rats-H & E 100 stains.
In lung tissue sections from diabetic rats, we found a disturbance of the normal structure of alveoli and alveoli, bronchioles, and interstitial arteries. The leading cause of disruption of the normal structure of lung tissue, significant alveolar collapse, was one of the most common observations on most slides and tissue sections (Figure 2B). In addition, the thickness of the epithelial layer covering the surface of the bronchioles increased. In several tissue sections, we observed the accumulation of homogeneous material in the arteries and exudative secretions between the alveoli. 3.3. The effect of diabetes on the expression of miRNA-155 and miRNA-133a in the lungs of rats.
According to the results, the expression level of the miRNA-133a gene was lower in diabetic rats than in control rats (Figure 3A) (p<0.05). However, the expression level of the miRNA-155 gene was higher in diabetic rats than in control rats (Figure 3B) (p<0.01).
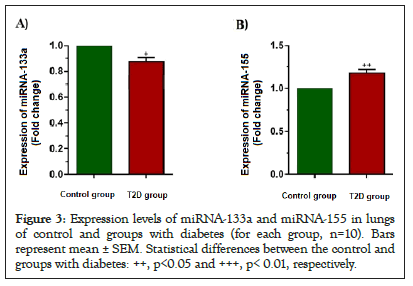
Figure 3: Expression levels of miRNA-133a and miRNA-155 in lungs of control and groups with diabetes (for each group, n=10). Bars represent mean ± SEM. Statistical differences between the control and groups with diabetes: ++, p<0.05 and +++, p< 0.01, respectively.
The prevalence of diabetes is increasing every day. Therefore, investigate its adverse effects, including the effects of diabetes on patient’s lungs. Previous studies have demonstrated changes in the lungs of patients with diabetes [24,25]. Therefore, we studied the lung tissue of rats to control the pulmonary changes in diabetes. In this study, we investigated the effects of diabetes on the expression of two genes, miRNA-155 and miRNA-133a, in the lung tissue of diabetic rats.
When one study examined the effects of diabetes on lung function, the results showed that the total lung capacity was lower in the diabetic group than in the nondiabetic group [26]. In another study examining histopathological changes in the lungs of diabetic rats, significant enlargement of the interalveolar septum was noted after four weeks, indicating cellular infiltration [27]. Eight weeks after the onset of diabetes, the collapse of many alveoli caused the lung tissue to lose its normal structure. In addition, an accumulation of collagen fibers was observed in the diabetic group compared with the control group [28]. In our study, the lungs of the diabetic and control groups were examined by light microscopy with hematoxylin and eosin staining. The lung tissue sections of the rats in the control group showed normal tissue structure. However, when we examined the lung tissue sections of the diabetic rats, we found disruption of the natural structure of the alveoli as well as the bronchioles and interstitial vessels. During the study, we did not mention the deposition of collagen and interstitial proteins because the study of collagen deposition requires Mason trichrome staining, which unfortunately was unavailable to our research team.
Expression of miRNA-133a in bronchial smooth muscle is markedly reduced in asthma patients, which increases RhoA levels and enhances bronchial responsiveness and sensitivity [29]. miRNA-133a plays an antifibrotic role in IPF by inhibiting myofibroblast differentiation and preventing lung fibrosis by inhibiting Transforming Growth Factor-β (TGF-β) [15]. In our study, miRNA-133a expression was lower in diabetic rats than in controls, which follows studies on diabetes. In our study, the expression of miRNA-155 was increased in the lungs of diabetic rats compared with control rats. A study investigating the effect of miRNA-155 on the pathogenesis of diabetes complications found that the expression of miRNA-155 was reduced in tissues other than the liver, including peripheral blood, heart, sciatica, and aorta of diabetic rats. This article also confirmed changes in the expression of this miRNA in diabetic rats [30]. In another study, the expression of miRNA-155 was also lower in diabetic rats [31]. However, these studies did not examine lung tissue. Thus, their results contradict our results but support the importance of this miRNA in diabetes.
Zhang, et al. also showed that a deficiency of miRNA-155 plays a protective role in preventing cardiac fibrosis in diabetic rats [32]. In another study, a group concluded that miRNA-155 reduced the prophylactic effect of TGF-β [33]. In a study by Kuroska, et al. [34], miRNA-155 increased collagen deposition and the production of TGF-β, this mediates pulmonary fibrosis. Moreover, Moses mentions that miRNA-155 increased collagen production mediated by TGF-β [35]. MiRNA-155 plays a vital role in the progression of systemic sclerosis. Its expression is markedly increased in the lungs of patients with systemic sclerosis, and miRNA-155 is an essential biomarker for the progression of pulmonary fibrosis in these patients. This effect could be an important target for treating this disease [36]. These articles follow our study, which found an increase in miRNA-155. Although we did not investigate the exact pathogenesis of miRNA-155 expression in diabetic lung lesions, previous studies suggest that miRNA-155 causes fibrosis and increased collagen deposition by acting on inflammatory and fibrotic factors. According to recent articles, using miRNAs as diagnostic biomarkers and therapeutic targets is strongly recommended. These miRNAs could be a good target for treatment, especially for preventing diabetic lung problems.
The prevalence of diabetes is increasing every day. Therefore, research into its side effects, including the effects of diabetes on patients' lungs, is essential. In this study, we found changes in the lung tissue of diabetic rats. We also found that the expression of miRNA-155 increased in the lungs of diabetic rats, and the expression of miRNA-133a decreased in the lungs of diabetic rats. Based on all these findings, we suggest that miRNA-155 is a significant cause of lung injury in diabetes when its expression is increased and that miRNA-133a can cause lung injury when its expression decreases. To further support these findings, we recommend conducting studies with more extensive samples and investigating changes in lung tissue by manipulating the expression of these genes to evaluate their therapeutic or prophylactic effects. Organ indices directly related to diabetes, such as the liver and kidney, can be evaluated to support diabetes. Therefore, we recommend that analysis of these organs be considered in future studies. We also recommend that future studies examine the effects of diabetes on the expression of genes and miRNAs at the proteomic level.
The authors declare there are no conflicts of interest.
The data and materials used in this study are available.
The Ethics Committee of the Tabriz University of Medical Sciences approved the study protocols following the Declaration of Helsinki.
This study was supported by a grant from Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1398.344).
Citation: Khezri M, Rahbarghazi R, Ahmadi M, Sandoghchian S, Nourazarian A, Shademan B, et al. (2023) Type 2 Diabetes and its Effects on MicroRNA-155 and MicroRNA-133a Expression in Lung Tissue of Male Rats. J Clin Chem Lab Med. 6:263.
Received: 27-Feb-2023, Manuscript No. JCCLM-22-20177; Editor assigned: 02-Mar-2023, Pre QC No. JCCLM-22-20177 (PQ); Reviewed: 16-Mar-2023, QC No. JCCLM-22-20177; Revised: 23-Mar-2023, Manuscript No. JCCLM-22-20177 (R); Published: 30-Mar-2023 , DOI: 10.35248/JCCLM.23.6.263
Copyright: © 2023 Khezri M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.