Virology & Mycology
Open Access
ISSN: 2161-0517
ISSN: 2161-0517
Research Article - (2022)Volume 11, Issue 4
The best delineated and well-studied plant viral insect vectors include aphids, whiteflies, leafhoppers, plant hoppers and trips. In addition for being the carriers of transmission, insects play a vital role in the infection cycle of many plant viruses. Plant viruses have developed a number of strategies to gain efficient transmission via insect vectors from one individual plant to the next. Most of them have adapted the capsid strategy (Cucumovirus, Crinivirus) and helper component strategies (Caulimovirus, Potyvirus) with variations between different virus genera. Consequently, there is necessity of protein–protein interface between the viruses and their specific vectors to determine acquisition from infected plants and their transmission to healthy hosts. With the development of molecular biology, mutagenesis and reverse genetics have facilitated the accurate identification of viral molecules that regulate the particular interaction with vectors. Therefore, the mechanisms that are responsible for the transmission of various viruses have been deciphered out with this achievement. Further exploration of the interactions responsible for behavioral changes in the insects might uncover the unknown factors that are involved in the process. The apparent specificity of these interactions in circulative and non-circulative plant virus transmission offers opportunities for disruption of vector population and virus transmission control. Keywords: Plant virus transmission; CMV; CaMV; Potyvirus; Insect vectors; Capsid
Plant virus transmission; CMV; CaMV; Potyvirus; Insect vectors; Capsid strategy; Helper strategy
Viruses are holo-parasites that reproduce by exploiting the cellular machinery of host. Biologically, a vision consists of an inner core of nucleic acid, usually wrapped in a protective protein or lipoprotein coat and competent to organize its own replication only within compatible host cells. More than 2000 virus species belonging to at least 21 viral families and 8 unassigned genera are adapted on plants, most of which cause destructive diseases of various commercial crops [1]. Therefore, 47 percent of the novel plant diseases are reported to be caused by viruses exclusively [2]. This data confers that plant infecting viruses are very successful pathogens and lead to the huge economic losses that are being reported continuously every year [3,4].
Since, viruses are totally dependent on the cellular network of hosts to complete their life cycle and transmit from one susceptible individual to the next. Therefore, they have to adapt to the characteristics of their hosts at various stages of their infection cycle (e.g. genome replication, protein translation, local cellular movement, and plant-to-plant transmission [5]. To get access into the host system viruses must cope with host characteristics that limit viral entrance and their transmission to other individuals [6].
Viruses have evolved distinct strategies to overcome the intact plant cuticle and the cell wall either by avoiding penetration into surface layers (i.e. in mechanical transmission, vegetative propagation transmission, seed transmission etc.) or by any method that needs penetration through an incision or puncture in the outer surface (in Insect and Arachnidan transmission, nematode transmission etc.). Although, some virus species (e.g. Potyvirus X and Tobamovirus) adopt an efficient strategy to avoid penetration into host cell outer surfaces but most of the plant viruses use extrinsic tools to disrupt the cell wall for accessing both the inoculation and acquisition processes through a vector. The most prominent and efficient vectors that are reported to transmit viruses from plant to plant include nematodes, fungi and plant-feeding arthropods, especially insects that we are going to discuss in this review.
Arthropods that transmit maximum plant viruses are aphids, leafhoppers, whiteflies, beetles, trips, mealy bugs, and mites [7]. Among them the most common are aphids with more than 200 species reported that act as plant virus vectors [8]. Out of nearly 550 vector-transmitted virus species recorded so far, 55 percent plant viruses are disseminated by aphids while as beetles and leafhoppers transmit 11% each [9]. The major genera that have been documented as viral vectors belong to genus Aphis, Myzus, Macrosiphum, Acyrthosiphon and in the subfamily Aphidinae.
Aphids have several features that incline them to act as successful viral carriers. The most important characteristic is their polyphagous nature. Aphids feed on wide domain of plants, including shrubs, vegetables, ornamentals and fruit crops. Therefore, wide domain of hosts for the aphids in turn broaden the host range of aphid mediated virus. A single green peach aphid (Myzus persicae) transmits almost 110 plant viruses [10]. In addition, aphids reproduce by parthenogenesis. A typical life cycle of an aphid involves the flightless females that give birth to female nymphs without involvement of males during the summer and produce sexual males and females in autumn [11]. The populations are thus increased in the geometric fashion during peak periods of crop growth that enhance virus dissemination to the larger number of plants. The third most important feature that helps aphids for being dominant virus vectors is the presence of piercing and sucking style for probing plant tissues. Because of this equipment an aphid is able either to acquire or to inoculate the virus from the plant cells. Therefore, the feeding behavior and host selection are considered as key elements in virus epidemiology [12].
Whiteflies that transmit 9 percent of plant viruses pose a major threat for spreading of devastative viral diseases among major agricultural crops. Nematodes are reported to carry 7 percent plant viruses while 5 percent are disseminated by fungi and Plasmodiophorids, and 2 percent by trips, mites and mealy bugs [13]. The brief description of insect vector families and their contribution in virus transmission is given in Table 1 [13,14] and shown in (Figure 1).
Table 1: Distribution of insect vector families and their contribution in virus transmission.
| Family | Common name of insect group | Number of vector species | Number of viruses transmitted |
|---|---|---|---|
| Delphacidea | Plant hopper | 28 | 24 |
| Cicadellidae | Leaf hopper | 49 | 31 |
| Aphididae | Aphid | 192 | 275 |
| Aleyroididae | Whitefly | 3 | 43 |
| Pseudococcidae | Mealy bug | 19 | 10 |
| Chrysomelidae | Leaf beetle | 48 | 30 |
| Cucurlionidae | Weevil | 10 | 4 |
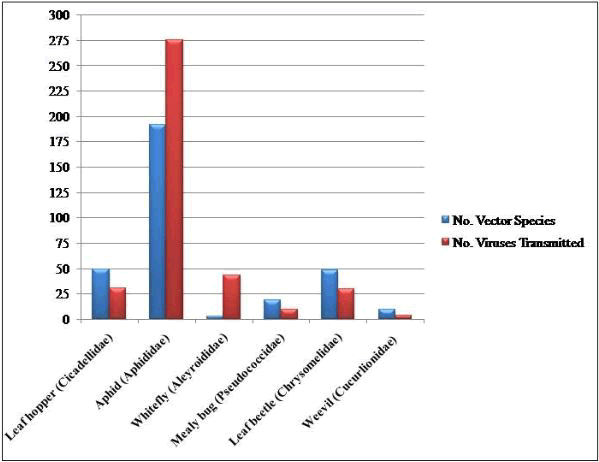
Figure 1: Graphical representation depicting the number of insect vector species and number of viruses transmitted by them.
The plant to plant movement via insect vectors is typically a multidimensional phenomenon that involves complex association between the virus, the vector, and the host plant, and impact of environmental factors. Most of the viruses show specificity with their vectors. Therefore, some viruses that are transmitted by one type of vector are not transmitted by any of the others. The vector specificity is not only at the family, genus, or species level but it is reported even at the biotype levels [15].
Stages in virus vector interactionVirus transmission cycle executed by an insect involves the transfer of visions from one infected host plant to many healthy plants. This transmission cycle is operated in three stages. The first step is acquisition phase during which the vector feeds on the infected plant and receives adequate viral load for transmission. Some viruses require the latent period during which the vector is not able to transmit it even after having sufficient viral load. The third stage, retention period is the span of time during which the vector remains infective and is able to transmit virus to others host plants [16].
On the basis of time requisition for each stage, the virus transmission by insects has been classified into three types: [6]
Non-persistent transmission or non-circulative viruses: In this mode of transmission, only less than a minute is requires for both acquisition and inoculation of viruses [3]. Latent period is absent and the entire transmission cycle is accomplished within a few minutes. Viruses carried in this way get attached inside the stylet (in aphids), hence known as ‘style-borne viruses’ (e.g. Potyvirus, Fabavirus, Cucumovirus etc.)
Semi-persistent transmission: In this mode of transmission, virus acquisition and inoculation requires prolonged duration than non-persistent transmission (minimum-15 minutes) [17]. The latent period is absent and the aphids are retained even up to two days [18]. These viruses get attached with the foregut of the insects, hence known as ‘foregut-borne viruses’ (e.g. Caulimovirus, Cloesterovirus, Waikavirus etc.)
Persistent transmission or circulative viruses: In this mode of transmission, virus acquisition and inoculation requires extended periods of plant contact. The viruses require latent period to cross the internal and external tissue barriers inside the insect [3]. Once this latent period is over, the vector can remain infective either throughout life i.e. (Persistent Propagative transmission) (e.g. Cytorhabdovirus, Fijivirus, Marafivirus etc.) or for a particular stage of life i.e. (Persistent Non-Propagative transmission) (e.g. Luteovirus, Nanovirus, Mastrevirus etc.). The brief description is given in the Table 2.
Table 2: Different modes of transmission with time varying stages.
| Transmission modes | Circulative | Non circulative | ||
|---|---|---|---|---|
| Propagative | Non propagative | Stylet borne | Foregut borne | |
| Acquisition time | Minutes-hours | Minutes-hours | Seconds-Minutes | Minutes-hours |
| Retention time | Days-Months | Days-Months | Seconds-Minutes | Minutes-hours |
| Latent time | Weeks | Hours-Days | Absent | Absent |
The primary difference in terms of viral transmission mechanism is whether ingested visions are circulative or non-circulative in the vector, a differentiation that depends on the retention time and/or retention site, as well as the cell-to-cell movement inside the aphid. Circulative viruses enter cells, traverse several membrane barriers, move through the vector hem lymph, and eventually emerge in the saliva of aphids. As discussed above, based on whether the acquired virus reproduces within the vector, circulative viruses are classed as circulative non-propagative or circulative propagative. However, non-circulative viruses have transient relationship with their vectors as they exclusively get attached within their mouthparts and/or foregut.
Non-circulative, style-borne transmission: Approximately, 300 plant viruses are disseminated by aphids in a non-circulative style borne mode [6]. The most important viral genera which show this type of transmission include Cucumovirus, Alfamovirus (Bromoviridae), Potyvirus (Potyviridae), Carlavirus (Flexiviridae), Fabavirus (Comoviridae), and Badnavirus (Caulimoviridae) [15]. These viruses are also known as non-persistent because of their less retention time for which an aphid remains viruliferous. The virus transmission in this mode is indicated by brief style penetrations (less than one minute) for rapid acquisition and inoculation of viruses [19]. While piercing the host tissue, the style does not normally enter beyond the epidermal cells, and once it penetrates the mesophyll and vascular tissue, the rate of transmission declines drastically [20]. After the acquisition, plant viruses are retained in the style.
In non-persistent viral transmission, there are two phases of interactions: virus retention at a particular site and escape of virus from that site [21]. Therefore, the viruses transmitted in this manner have a basic structure of nucleic acid encapsulated in compact icosahedral or rod shaped coat proteins. As a result, the interactions of the cells inside vectors are limited to the capsid protein. However, the major quandary is that the binding of visions within the vector must be promptly reversible. It is pertinent to mention that the food and salivary canals merge at apical end of the aphid style; therefore, salivation may facilitate to the release of bound visions and enhance their entry into plant cells [22]. In addition, the molting of an aphid leads to shedding of cuticle lining on the salivary and food canal resulting in the loss of viral load [23].
Consequently, two types of contacts have been observed during the retention phase: one in which the viral capsid seems to engage directly with the retention site in aphid (Capsid strategy) and another wherein a nonstructural virus-encoded protein is required (Helper strategy). This nonstructural protein is termed a helper component, helper factor, or aphid transmission factor [6].
Capsid strategy: In this strategy, the virus directly gets attached with the vector via its coat protein. This approach has been proven in the viruses including Cucumovirus, Coronavirus, Carla virus and Alfamovirus genera. The development of in-vitro aphid-transmission assay by has been a milestone towards this research area. The authors provided the evidence that the purified viruses can be readily transmitted [24-27].
Capsid strategy in cucumber mosaic virus: In an elegant experiment, showed that the Cucumber Mosaic Virus can be reconstituted into biologically active form by recombining its nucleic acids and Coat Proteins (CP). While making the mixtures of nucleic acid extracted from Poorly Aphid Transmissible isolates (PAT) and coat protein from Highly Aphid Transmissible (HAT) isolates of CMV which revealed effective transmission, confirmed that the virus transmission efficiency was exclusively controlled by the CP. Consequently, determined the organization of CMV at 3.2 Å resolutions and identified a loop (the βH-βI loop corresponding to Amino Acids (AA) 191 to 198) in CP sequences of Cucumoviruses. In addition, it was confirmed that the loop was conserved and present on the inner surface of aphid style. The βH-βI loop carries high negative charge with residues 192 and 198 important for metal ion binding. The scientists also suggested that the attached ion may affect charges on the surface of vision, which could be crucial for virus-vector interaction. However, the results from these experiments could not explain the transmission of several other viruses like Potyvirus and Caulimovirus.
Helper strategy: In this approach, the virus-vector interface is facilitated by a non-structural protein expressed by the virus termed as "Helper Component." The viral genera Potyvirus and Caulimovirus have been reported to adapt this strategy. The notion of helper strategy was introduced by Kassanis and Govier in 1968 when they identified the missing element that was evidently lost during viral purification. The theory was established on the restoration of transmissibility of a non-transmissible Potyvirus variant following by co-infection with its transmissible strain [28]. The authors attributed this restoration to a substance derived from plants infected by the transmissible isolate. This compound was termed as ‘Helper Component’ (HC).The molecular basis of helper component was described by a hypothesis later termed as the ‘bridge hypothesis’ [29]. The hypothesis proposed that the two independent interacting domains would link the CP on one side and the putative attachment sites within the insect (circular lining of common duct) on the other.
Helper strategy in pot virus: Potyviruses are filamentous flexuous, positive ssRNA viruses having 10kb genome size covalently attached 5'-terminal viral protein (VPg) and a 3'-terminal polyadenylated tail [30]. Three viral-encoded proteases split a single Open Reading Frame (ORF) encoding a polyprotein into ten functional products [31,32] as shown in (Figure 2). Commensurate with several experiments like as protein purification, immunoprecipitation, and transmission assays, the N-terminal portion of the Pot virus is transcribed into a polyprotein that promotes the aphid-mediated transmission of visions [33].
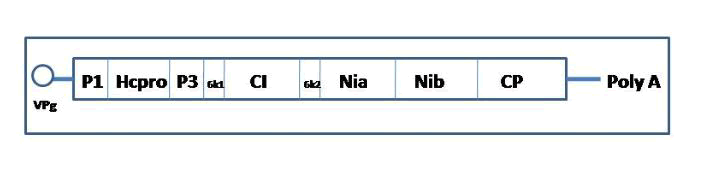
Figure 2: Genome organization of and protein products Potyvirus.
This polypeptide gets cleaved off at its 3' end from the polyprotein by its autocatalytic protease activity hence, designated as HC-Pro (Helper Component-Protease). According to the biologically active form of HC-Pro is a homodimeric, which is a dimer made up of two subunits of HC-Pro molecules joined by a hinge at N' of one molecule to C' of the other. To explain how the vision is bound inside the aphid style, two theories have been intended. According to one theory, two HC-Pro molecules are joined in such a way that one molecule is connected to a "receptor" on the style and the other molecule is attached to the coat protein component. Pursuant to the second opinion, both HC-Pro molecules bind laterally to the receptor on style and coat protein subunits of vision as shown in (Figure 3).
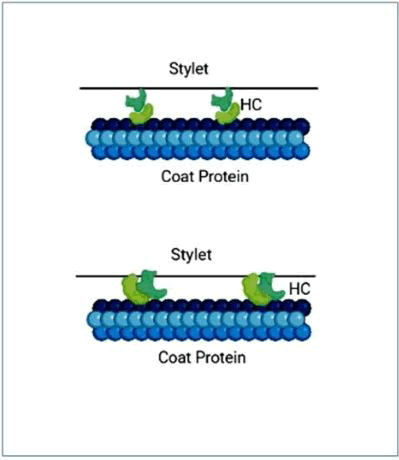
Figure 3: Schematic diagram showing two different ways of HC-pro connected with aphid stylet.
The first explicit declaration that HC-Pro acts in line with the bridge hypothesis was attempted using transmission electron microscopy [34]. Stated that a peptide domain, the four-residue motif "KITC" (lysine-isoleucine-threonine-cysteine), is present at the N-terminal end of the HC-Pro sequence that is required for the interaction with an unknown aphid receptor. Additionally, at the C-terminal end of the HC-Pro sequence holds a “PTK” (proline-lysine-threonine) motif for effective interaction in between the HC-Pro and the “DAG” (aspartic acid-alanine-glycine) domain located at the C-terminus of the CP. Aside from its transmission role, HC-Pro has been reported to possess many other properties and/ or functional domains. The HC-Pro has recently been characterized as a gene silence suppressor [35,36] and its role in the necrotic response to viral infection of host [37]. The HC-Pro protein also possess a zinc-finger motif linked to the synergistic effects with other viruses [38] along with nucleic acid binding domains “IGN” [39] and “CC”/“SC” peptide domains that are important in the viral RNA replication and viral systemic movement inside the plant, respectively [40]. The component appears to play a role in virus movement by the way of plasmodesmata [41] as well as access to plant vasculature [42] for systemic infection.
Helper strategy in Caulimovirus: CaMV is a circular dsDNA virus with an 8kbps genome and seven ORF’s, six of which have products P1–P6 [43] . Out of these, P2, P3, and P4 are three of the six proteins that have been linked to transmission [44]. P2 is associated with the glycosylated protein embedded in chitin matrix through its N´-terminus and its C´-terminus binds to N'-terminal area of P3, which in turn is anchored within the vision capsid shell, where it forms a tetramer with P4 that forms the capsules of capsid [45] as shown in (Figure 4).
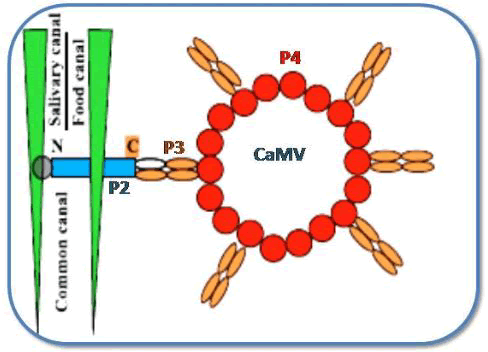
Figure 4: Mechanism for the interaction between CaMV, proteins and vector.
Once CaMV replicates inside infected plant cells, it creates electron dense separate viroplasms, encoded by the P6 protein, in which offspring visions may be seen under light and electron microscopy [46]. Prior to the acquisition of virus by its aphid vector, CaMV responds the stimulus of vector probing by forming the electron-lucent inclusion bodies [47]. The CaMV-encoded P2 protein is the main component of these elIBs. Since these inclusion bodies are specifically meant for transmission. Therefore, these are also called as “Transmission bodies” (TBs). CaMV-induced TBs "feel" cell injury and dissociate and re-distribute P2 onto cellular microtubules as soon as they are probed. This capability of recognizing the aphid activity and elicit a response to it by a virus is called as “Virus perception behavior” [48]. The P2 binds to its receptor inside the style, whereas P3 dissolves in the free confirmation. Due to the strong affinity between P2 and P3, the aphid is loaded with P2 and acquires P3 vision complexes from viral factories [49]. Finally, P2 is transferred back into TBs when aphids depart the leaves [50] used high-resolution methods to discover the CaMV-encoded P2 binding sites in aphid style. These experiments guided to the emergence of the important findings: I) The styles containing Green Fluorescent Protein (GFP) fused to CaMV P2 protein (HC) showed fluorescence only when evaluated with P2: GFP fusion proteins, not when tested with GFP alone. II) The P2: GFP was found to bind to styles of vector-aphids but not to non-vector aphid styles. III) A mutant P2: GFP (P2Rev5: GFP) with a Q to Y mutation at amino acid 6 in P2 did not bind to styles in vector-aphid. However, as previously described, this mutation causes loss of transmission by inhibiting the bioactive form of P2 [51] and IV) The authors confirmed the most important fact: P2: GFP fluorescence was localized to a distinctive and microscopic region at the style tip, later designated as ‘acrostyle’ (Figure 5) and recommended that because of its uniqueness for being an ideal location for receptors of CaMV and possibly to other non-circulating viruses".
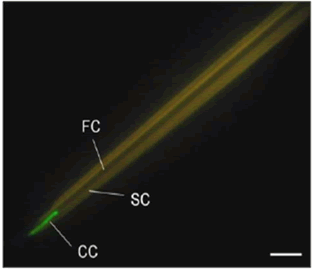
Figure 5: A. Interaction assay between isolated stylet of A. pisum and the P2 protein (HC) of CaMV fused to GPF.
Non-circulative, foregut-borne transmissionDifferent viruses transmitted by aphids, whiteflies, and leafhoppers have a non-circulative, semi-persistent manner of transmission. Viruses that adopt this mode of transmission are kept by their vectors for hour’s way longer than style-borne viruses [52]. The acquisition time varies from minutes to hours and latent period is totally absent. Feeding, rather than probing, appears to be the key to efficient virus acquisition. Post-Acquisition Starvation (PAS) does not affect transmission efficiency. The infective viruses are maintained in chitin-lined regions of the anterior foregut that are lost over the insect mounting. Closterovirus (Closteroviridae), Coronavirus (Closteroviridae), Sequivirus (Sequiviridae), and Waikavirus (Sequiviridae) are the four predominant viral genera that are semi-persistently transmitted through the foregut.
Minor capsid strategy utilized in Lettuce Infectious Yellows Virus (LIYV): The visions of Lettuce Infectious Yellows (LIYV, genus Coronavirus, family Closteroviridae) are filamentous and possess only four LIYV-encoded proteins (CP, CPm, HSP70h, P59) [53]. However, the minor Capsid Protein (CPm) and CP (major capsid protein) are the two most important vision proteins. The CPm only covered about 10 percent of the vision from one terminal indicating that versions were morphologically polar [54]. The pure visions could be obtained in vitro thereafter transferred to the plants by whiteflies (BemesiaTabaco). Exploiting the in vitro transmission experiments, reported the identification of LIYV- encoded protein responsible for its transmission and the place of retention in its vector. As the purified viruses were transmissible, the isolated LIYV visions were treated with antibodies specific to each of the four vision proteins independently. The supernatant was then centrifuged and fed to the vector whiteflies. Antibodies against the LIYV CPm were found to be the only ones that prevented B. tabaci from acquiring and transmitting LIYV to plants. The fluorescent antibodies detected in the anterior foregut were seen using field fluorescence microscopy and confocal laser scanning microscopy [55,56]. These results corroborate the theory that the CPm is a LIYV-encoded protein complicated in transmission of LIYV via B. tabaci.
Circulative and propagative transmissionThe word ‘‘Circulative’’ coined and this term applies to those viruses that spend part of their life cycle inside the body of vector. Circulative propagative transmission relates preponderantly vector-transmitted vertebrate contaminating viruses (Parvoviruses). These viruses organize their own replication that is totally dependent on the vector protein synthesizing machinery inside the vector's body. The important viral genera transmitted via this mode include members of Rhabdoviridae (Cytorhabdovirus, Nucleorhabdovirus) and Reoviridae (Fijivirus, Phytoreovirus, and Oryzavirus). The major vectors of these persistent-propagative viruses are plant hopper (Hemiptera: Delphacidae) or leafhopper (Hemiptera: Cicadellidae). The major viral diseases along with their vectors discussed under this group include Rice Dwarf Virus (Nephotettix cincticeps), Tomato Spotted Wilt Virus (F. occidentalis), Rice Grassy Stunt Virus (Nilaparvata. lugens), and Rice Stripe Virus (Laodelphax striatellus). In their transmission cycle, acquisition time varies from hours-days while as retention time is from weeks- months. These viruses show the latent period up to several weeks during which the visions cross the membrane barriers of vector tissue and perform replication inside their cells [57,58].
The vector mediated transmittance of plant-infecting Reoviruses is adequately marked owing to availability of diverse tools including Vector Cell Monolayers (VCM) and RNA interference. RNA interference helps in understanding viral gene activity in segmented viral genomes in spite of the absence of an infective clone system, while VCMs are effective in researching virus infection at the cellular level with a synchronous infection. The vectors are also susceptible to RNAi, which can be used to reduce the extent of transcripts of possible host proteins engaged in the virus infection cycle [59].
Mechanism adopted by Rice Dwarf Virus (RDV): The foremost illustrated virus–vector system across the family Phytoreovirus is the Rice Dwarf Virus (RDV) along with its carrier Nephotettixcincticeps. Virus after acquisition passes through the alimentary canal and reaches to the mid gut. To get entry into the gut cells and start replication, the viruses need to overcome the plasma lemma and basal lamina of the gut epithelial tissue [60] (Figure 6).
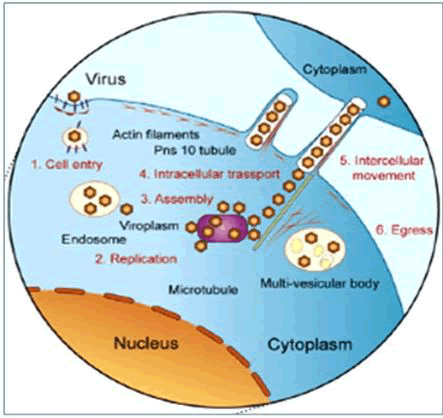
Figure 6: The model of Reovirus infection cycle and their cell to cell movement via tubular structures made of virus nonstructural proteins.
Entry into gut cells: Circulative viruses have adapted the endocytic and exotic transport process (transcytosis) to overcome the cellular barriers. The visions are picked up by clathrin-mediated endocytosis when they get attached to gut cells [61]. Researches on vector cell monolayers (VCMs) indicated that the viral structural capsid protein P2, a small component of RDV's outer capsid, is accountable for the virus's initial entry into VCMs and midget tissues [62] (Figure-6).
Replication cycle and vision assembly: The P2 protein is a protein complex that aids in the separation of vision from the endocytic vesicle, afterwards the reproduction cycle of virus begins. After replication, progeny visions assemble inside cells and are contained moreover inside the tubules in a tight row or accumulated in multiple vesicular bodies inside these cells [63]. Pns10, a non-structural protein, is the major component of RDV-induced tubule formations [64] (Figure-6).
Intercellular transport: The RDV cell to cell movement is intervened via Pns10 tubules which are coupled with actin-based filo podia that extend from the surface of infected cells and are competent enough to penetrate through neighboring cells [65]. In addition, the endomembrane system and myosin motors connected with actin filaments are necessary for RDV transport via tubules to neighboring cells. The unique interaction within Pns10 and cytoplasmic actin of the vector (Nephotettixcincticeps) is a determinant of vector specificity, according to [66]. Viral particles sprout through the plasma membrane and get collected in intercellular gaps of salivary ducts, where from they are injected into new hosts [67] (Figure 6).
Some other propagative viruses have established diverse techniques to spread inside the body parts of the vector. During the insect stages when the midget and salivary tissues are in close contact, tospoviruses (Tomato spotted wilt virus/TSWV) inside its vector cells Frankliniellaoccidentalis (western flower Thrip) disperse out from the midget to the salivary glands through connective tissue of the salivary glands, ligaments, and/or directly between tissues [68]. Viruses are not observed in tubular structures inside cells despite having the capability to induce the tubule formation inside its vectors [69]. As compared to RDV, Fijivirus, Southern Rice Black-Streaked Dwarf Virus (SRBSDV) form tubules comprised of the P7-1 protein inside the vector Sogatellafurcifera that are engaged in virus escape over the basal lamina [70]. Consequently, the latent duration for SRBSDV is shorter (6 to 9 days) than the latent period for RDV which varies from 2 to 3 weeks [71].
Circulative, non-propagative transmission: In case of circulative, non-propagative mode of transmission, the viruses do not spread inside their vectors body. As a result, no replication will take place in any of the vision-bearing cells. Consequently, these viruses require limited latent periods varying from hours to days as compared to propagative viruses. However, all ways of transmission are identical in as the visions pass through the gut epithelium, enter the haemocoel, and then pass via salivary gland cells so as to get transferred through the salivary ducts. These viruses are only found in gut and Auxiliary Salivary Gland (ASG) cells, virions will not be found in other cell types. The acquisition period is specific to virus-vector pair and may vary from hours to several days. Vectors may retain viruses up to several weeks. The most important plant viral genera transmitted via non-propagative mode include the Luteoviridae, Gimmiviridae and Nanoviridae [72]. These viruses are phloem-limited and hence carried by phloem limited aphids. Nano viruses are small, 6-8 icosahedral particles, each containing small circular DNA molecules as its genome [72] while as lute viruses are icosahedral particles with mono partite RNA molecule as their genome. The transmission of Luteovirids via aphids is better understood. It entails a high number and variety of proteins derived from numerous sources including virus, insect vector, bacteria, and host plant.
Mechanism adopted by Luteoviruses: The luteovirion structure is made up of the Capsid Protein (CP) and CP-Read through Protein (CP-RTP) that serves as a minor component (Figure 7). The Cp-Read Through Protein (CP-RTP) is produced by translational read through of the CP stop codon, causing C-terminal extension of the CP. Most of the studies held on these proteins have confirmed their role in virus acquisition during viral disease transmission [73]. Virions can still transcytose to haemocoel in the absence of RTP, signifying that the CP is adequate to deliver the virus to the haemocoel. The RTP's N-terminal domain is largely conserved and essential for its connection with the ASG, whereas the C-terminal region is variable and implicated in plant accumulation and tissue tropism [74].
The following are the two primary processes in the transmission of lute viruses (non-propagative viruses):
Receptor mediated endocytosis inside the hindgut: The viruses get enclosed in the coated pits of Apical Plasma Lemma (APL) and bud-off from it to form “Virus Containing Coated Vesicle” (VCCV) [75]. These CV’s are then transported to receptosomes to accumulate the virus load. Tubular vesicles with linear aggregation of virus on receptosomes are formed and carried to the Basal Plasma Lemma (BPL), whereby their union allows release of the virus from the cell to diffuse into haemocoel [76]. Those virus particles that remain in the lysosome are likely destroyed as the empty receptosome (endosomes) grow into lysosomes (Figure 7).
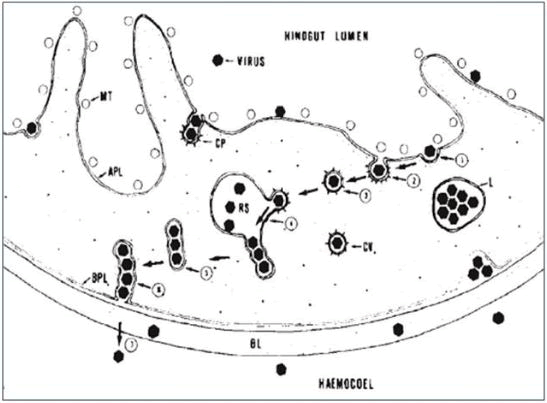
Figure 7: Crossing of Apical Plasmalemma (APL) and Basal Plasmalemma (BPL) of hindgut by luteoviruses so as to diffuse into haemocoel.
Lute virus interactions with Accessory Salivary Glands (ASG): When lute viruses via haemocoel reach to the ASG they first come across the extracellular Basal Lamina (BL) that acts as a selective barrier and allows the diffusion of specific lute viruses. Viruses which cross the BL encounter a second selection barrier at the Basal Plasma Lemma (BPL) [77]. Virus particles that are not addressed by putative virus receptors at the BPL remain outside the cell within per cellular space, whereas those that are recognized by coated pits at the BPL are encysted and deposited in Tubular Vesicles (TV) inside the Cytoplasm (C). Those TVs that are close to the microvilli-lined canals of (APL) form Coated Vesicles (CVs) that encapsulating individual virions. Such CVs get fused with the APL by forming coated pits and viruses are released into canal lumen to carry lute virus and salivary secretions out from the aphid together with salivary secretions [78] (Figure 8).
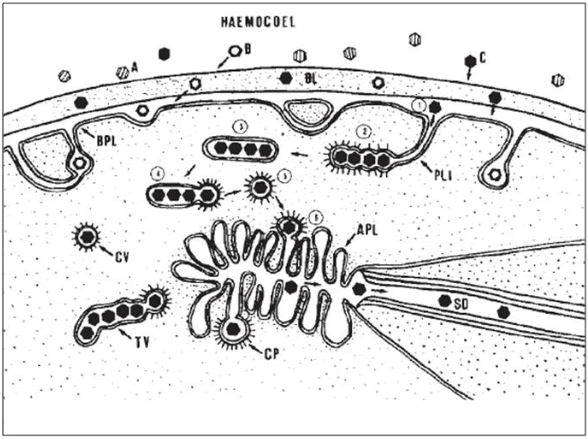
Figure 8: Crossing of Basal lamina (BL), Basal Plasmalemma (BPL), and Apical Plasmalemma (APL) of Accessory Salivary Gland (ASG) by luteoviruses to reach to Salivary Ducts (SD).
Transmission is a vital step in the natural cycle for many plant viruses to insure their survival and maintenance after spreading from one host to next via their vectors. Different viral groups have adapted different mechanisms for transmission which show independence on the kind of genome, particle form or viral protein expression strategy. The development of molecular biology, mutagenesis and reverse genetics has enabled for the exact identification of viral components that regulate virus-vector interactions. Protein–protein interactions among the plant viruses including their insect vectors frame a vital molecular interface that determines virus uptake from infected host plants and their transmission to new hosts. Viruses are bound to conserve these genomic regions encoding their transmission proteins during their replication process for their continuity in nature. The experiments have confirmed that mutations in transmission-protein encoding genes will lead to complete loss of transmission property in viruses. Other gene products that have a direct or indirect effect on vector behavior in respect to the infected plant host contribute to increased transmission efficiency and dissemination. Further research into the processes underpinning the insect's behavioral changes could reveal which elements are at play. Disentangling the biochemical and molecular interactions in between a virus or its vector will lead to the discovery of new prospects for developing novel plant viral disease management methods.
Citation: Mubashir SS (2022) Understanding the Molecular Prospective of Plant Virus Transmission via Insect Vectors. Virol Mycol. 11:236
Received: 23-Feb-2022, Manuscript No. VMID-22-16000; Editor assigned: 25-Feb-2022, Pre QC No. VMID-22-16000; Reviewed: 11-Mar-2022, QC No. VMID-22-16000; Revised: 25-Apr-2022, Manuscript No. VMID-22-16000; Published: 02-May-2022 , DOI: 10.35248/2161-0517.22.11.236
Copyright: © 2022 Mubashir SS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.