Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Review Article - (2021)
Retinitis Pigmentosa (RP) belongs to the group of inherited degenerative retinal dystrophies affecting the photoreceptors, particularly rods and subsequently cones. They are usually bilateral but there can be asymmetrical presentation too. Unilateral Retinitis Pigmentosa (URP) in both adult and pediatric population or a genetic predisposition for the same has not been clearly established in the literature. Unilateral variety however has mimickers in the form of infectious, inflammatory, traumatic, vascular and neoplastic etiologies. This review article would highlight the possible etiopathogenesis, molecular genetics, multimodal imaging and differential diagnosis of unilateral RP. Med Line and Pub Med search was done pertaining to Unilateral Retinitis Pigmentosa (URP) unilateral pigmentary retinopathy, and genetics, electrophysiology, autofluorescence, optical coherence tomography, microperimetry and differential diagnosis, all related to unilateral retinitis pigmentosa.
Unilateral retinitis pigmentosa; Genetics; Multimodal investigations; Differential diagnosis
Retinitis Pigmentosa (RP) is a form of hereditary degenerative dystrophy that involves the photoreceptors which leads to progressive damage, atrophy and cell death of the photoreceptors. Rods are affected initially and subsequently in advanced stages, degeneration of cones and retinal pigment epithelium ensue. This is a bilateral symmetrical condition that presents as waxy disc pallor, retinal arterial attenuation and bone spicule changes in all the retinal quadrants. The usual symptoms are decreased night vision (nyctalopia) and decreased peripheral field of vision in a concentric manner. Central vision loss occurs in the later stage of the disease and may be due to the disease process itself or development of posterior subcapsular cataract and cystoid macular edema. The hereditary pattern of inheritance is Autosomal Dominant (AD), Autosomal Recessive (AR) or X-linked (XL) and can be sporadic too. RP sine pigmento is a form of RP with the absence of typical bone spicules [1-3].
Francois et al. put forth the following criteria for the diagnosis of Unilateral Retinitis Pigmentosa (URP) [4],
• The involved or the affected eye has the classical features of RP.
• The fellow eye does not show feature of involvement and with normal fields and full filed Electroretinogram (ERG).
• It is mandatory to follow up the fellow normal eye for more than 5 years to rule out delayed involvement.
• No secondary causes of pigmentary retinopathy like infection, inflammation, trauma, vascular occlusion, drug toxicity that would mimic URP should be present.
URP is a rare condition but unilateral RP sine pigmento is even rarer than the pigmentary RP. Jacobson et al. [5] and Pearlman et al. [6] reported one case each of URP without pigmentation, but presence of arteriolar attenuation and minimal disc pallor that showed absent or reduced ‘b’ wave response in ERG, absent rod curve in dark adaptometry, constricted visual field with preserved central visual acuity and abnormal Electrooculogram (EOG). The predominant cause of central retinal artery occlusion was ruled out from the history and ensuing investigations. The other eye in both cases was normal and showed normal ERG response [5,6].
Other secondary causes of URP like trauma, infection, inflammation, autoimmune and cancer induced retinopathy and drug toxicity should be considered in the differential diagnosis.
Etiopathogenesis
URP or asymmetrical RP has not been reported much in the literature. This form of RP does not fit into the usual genetic variety and is hypothesized to be due to either mosaicism wherein the mutation affects only few cells or somatic mutation instead of germline mutation. The possibility of any person developing URP or asymmetric RP in such a scenario is based on whether the affected cells become retina or retinal pigment epithelium or transform into bone or muscle [7-9].
Most of the cases that are reported in the literature by various authors belong to this category.
Molecular genetics and familial predisposition
Marsiglia et al. [10] reported mutation of USH2AW4149R in 1/5 patients with URP and proposed the possible mechanism of somatic mutation or mosaicism for the asymmetric, unilateral or partial involvement of the genetic disease. However, he emphasized on the importance of imaging and functional testing and long term follow up of these patients. There was no family history of RP in any patient and other etiologies were ruled out.
Mukopadhyay et al. [11] reported mutation of p.R677X of RP1 gene, one of the most common causes for AD RP (germline mutation). Other eye was normal and he proposed either somatic or mosaicism due to embryonic mutation at this locus as the possible cause for unilaterality. Family history was positive for AD inheritance.
Farrell et al. [12] reported 5% of URP cases in their study. Familial forms of URP were reported and majority of them showed AD inheritance and one with AR pattern. There were no cases of XL and bilateral disease was present in all the affected relatives. The increased rate of URP may have been due to the study being conducted at a tertiary center with lot of scope for unusual cases.
Koenekoop et al. [13] reported asymmetric pattern of the disease in a female carrier of XLRP who exhibited mild disease due to random inactivation of the X-chromosome and severe disease in the fellow eye due to non-random inactivation of Xchromosome.
Sim et al. [14] reported mutation of CLRN1 and the proposed mechanism was that the mutation may have happened at 2 cell stage before embryogenesis that may have caused left-right segregation at the 8 cell stage and URP or this finding may have been coincidental.
Errera et al. [9] in his series reported 2 patients with genetic predisposition, one was a carrier of X-Linked RP and the other had a positive family history and a twin with bilateral disease and confirmed mutation.
Clinical findings
URP presents with history of nyctalopia and clinical findings in the form of waxy disc pallor with retinal arterial attenuation and bone spicules predominantly in the mid far peripheral fundus (Figure 1). Posterior sub-capsular cataract and Cystoid Macular Edema (CME), epiretinal membrane, full thickness or lamellar hole may be accompanying the classical fundus features and cause reduction of central vision [15].
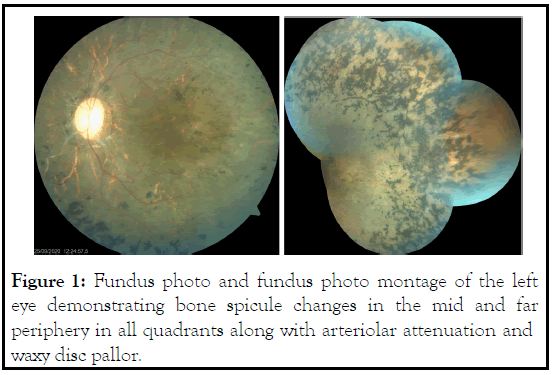
Figure 1: Fundus photo and fundus photo montage of the left eye demonstrating bone spicule changes in the mid and far periphery in all quadrants along with arteriolar attenuation and waxy disc pallor.
Multimodal investigations
Fundus autofluorescence (FAF): FAF shows hypo autofluorescence in the area of Retinal Pigment Epithelial (RPE) atrophy. This hypo autofluorescence extends to mid and far periphery corresponding to the area of RPE atrophy or degeneration and is evidenced by concentric fields. Hyper autofluorescence is seen around the macula and this corresponds to increased deposition of lipofuscin and other fluorophores following degeneration of photoreceptors. Area of preserved vision is seen corresponding to the inner border of the ring. Area outside this hyper auto fluorescent zone shows severely affected visual function. Disease progression can be monitored by the size of the ring [16-18].
Duncker et al. [19] compared Near Infrared AF (NIR) and Short Wavelength (SW) AF in RP and concluded that NIR-AF showed better contrast between the inner and outer rings when compared to SW-AF and thus help in estimating disease progression and delineating available healthy retina.
Optical Coherence Tomography (OCT): This can be used as an indicator of disease progression. The integrity of photoreceptor Inner Segment/Outer Segment (IS/OS) junction along with External Limiting Membrane (ELM) is correlated with visual acuity and visual field preservation. Loss of ELM along with continued absence of IS/OS junction is associated with disease progression and declining visual acuity [20-22].
Electroretinogram (ERG): It is not only helpful in monitoring disease progression but in recognizing early photoreceptor loss in the other eye in cases of asymmetrical or URP. Full field ERG measures the function of entire retina and is of immense use in cases of RP. It shows severe rod damage (scotopic response) and marked delay in cone flicker timing. This helps us differentiate between dystrophy and acquired cause of pigmentary retinopathy wherein there is only minimal delay in cone flicker timing [7,9,10,12,13].
Multifocal ERG is helpful in delineating the functional areas amidst non-functional areas in advanced stage of RP. Multifocal ERG shows gradual reduction and loss of amplitude outside the perimacular area. Central retinal function may get affected in advanced cases due to progressive degeneration as a part of the disease process. Electro-Oculogram has been shown to be abnormal in those with retinal dystrophies and particularly RP [23,24].
Visual fields: Humphrey perimetry shows mid peripheral ring scotoma or central tubular field.
Microperimetry: Assesses the central retinal function in RP cases by eliminating fixation losses, intra and inter observer variation and removing inability to testing consistent retinal points within and between the examinations [25].
Differential diagnosis
Other etiologies of URP or asymmetric RP include [9,12,26-28]
1. Severe commotio retina following blunt trauma, Siderosis bulbi following retained intraocular iron foreign body
2. Old retinal detachment, laser scars
3. Infection–Congenital Rubella retinopathy, syphilitic retinopathy, Lyme retinopathy, Diffuse Unilateral Subacute Neuroretinitis (DUSN), Toxoplasma, Cytomegalovirus
4. Inflammation–Retinal vasculitis, old posterior uveitis, Pars Planitis
5. Autoimmune disease and Neoplasm– Autoimmune Retinopathy (AIR), Cancer Associated Retinopathy (CAR), Acute Zonal Occult Outer Retinopathy (AZOOR)
6. Occlusive vascular disease (central retinal artery occlusion or ophthalmic artery occlusion)
7. Drug toxicity–Chloroquine/Hydroxychloroquine, Thioridazine toxicity, Chlorpromazine
8. Carriers of mutation of X-linked genes RP2 and RP3.
URP or asymmetrical RP is presumed to be due to mosaicism or somatic mutation. This theory might raise the question of whether it can cause inheritance, and the answer is that it can, if the mutation occurs during the embryogenesis period and affects those cells representing retina or RPE, though the risk is low [7-9]. It presents with the usual clinical features of bilateral RP but can also present as sine pigmento [5,6]. URP patients usually present late to the ophthalmologist when compared to those with bilateral presentation because of the fellow eye being normal. Francois et al. have clearly defined the criteria for unilateral RP [4].
Investigations include ERG which shows extinguished scotopic response and marked delay in cone flicker timing. Visual fields show mid peripheral ring scotoma or a central tubular field. Areas of retinal atrophy are marked as hypo auto-fluorescence and hyper auto-fluorescence denotes a small area of functional retina. ERG becomes the mainstay in following up these patients on a long term basis. It also helps us in identifying the fellow eye involvement at an early stage.
Differential diagnosis of URP includes traumatic cases as reported by Cogan et al. [29]. Inflammatory causes wherein other features of inflammation like keratic precipitates, posterior synechae, anterior chamber cells and flare or vitreous cells of evidence of vasculitis should be ruled out. Conditions like parsplanitis may trigger pigmentary changes mimicking RP. Routine and specific blood investigations are required to rule out inflammatory etiology [9,12,26-28].
Congenital causes like Rubella retinopathy cannot be diagnosed using serological investigations. It may not be appropriate to make a diagnosis of Rubella retinopathy in patients who present later in life. Clinically, AZOOR is characterized by sudden onset of scotoma usually following a viral prodrome and associated with photopsia. AZOOR shows abnormal 3 zonal autofluorescence in the peripapillary area and the corresponding OCT shows loss of outer retinal segments. Pigmentation in cases other than true RP is patchy and typical bone spicule formation and distribution may not be present. Cancer associated retinopathy presents with vision loss over weeks or months instead of years as in URP and shows anti-recoverin antibodies and is usually bilateral [9,12,26-28].
Potsidis et al. [30] reported that mean annual change of visual field area in the affected eyes was -4.9%, ERG amplitude with 0.5 Hz flashes was -4.7%, ERG amplitude with 30 Hz flashes was -4.6%. All these changes were faster in the affected eyes than in the fellow eyes, those with age>35 years than in younger patients and with an initial cone implicit time ≥ 40 ms. Follow up of fellow eye is extremely important to rule out asymmetry of the disease. Farrell et al. [12] followed up 2 cases for 8 and 14 years and did not find any evidence of disease in the fellow eye, same as Weller et al. [28] who followed up for 30 years without any evidence. However, Gauvin et al. [31] reported bilateral involvement after following up for nearly 30 years.
There is no specific treatment for URP. Genetic counselling can be done only in cases of genetic predisposition. Cataract and CME can be treated accordingly. CME in RP responds to topical carbonic anhydrase inhibitors when compared to that in other etiologies [26].
Low vision aids can be given to help with their daily needs. Vitamin A therapy and anti-oxidants are controversial and more studies are required to prove its efficacy. Gene therapy is still in the developmental stage and hence studies are required for further validation [26].
Thus, URP should follow the classical diagnostic criteria with the fellow eye being normal both clinically and electrophysiologically. ERG, FAF and OCT can be used to prognosticate and to look for early involvement of the fellow eye. URP mimickers should be borne in mid before making an actual diagnosis of URP. Though there is no permanent treatment, gene therapy is under way and conservative measures can be tried.
Citation: Priya RC (2021) Unilateral Retinitis Pigmentosa: A Review. J Clin Exp Ophthalmol. S18:003.
Received: 20-Sep-2021 Accepted: 04-Oct-2021 Published: 11-Oct-2021 , DOI: 10.35248/2155-9570.21.s18.003
Copyright: © 2021 Priya RC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.