Pancreatic Disorders & Therapy
Open Access
ISSN: 2165-7092
ISSN: 2165-7092
Review Article - (2021)Volume 11, Issue 4
Type2 Diabetes mellitus (T2D) refers to a syndrome that by definition is secondary to numerous extents of βcells failure in addition to reduction in insulin sensitivity. Despite, a lot of metabolic Impairment, most patients are classified as either presenting with T1D or T2D. Recently Ahlqvist et al. posited a new system of classification for adult onset disease, keeping in view the heterogenic metabolic phenotypes of this disease. This new classification system might possess the potential for utilization for greater individualization of treatment depending on the underlying metabolic Impairments in this disease; despite no existing medication studies have developed data to validate this claim. Thus here we provide a brief introduction on the etiopathogenesis with regard to T2D as well as in patients acquiring Diabetes at adult age, besides summarize the evolution of classification systems including one we had earlier provided. Subsequently we try to review the actions of various antidiabetic agents on insulin sensitivity along with β cell function in addition to the posited approaches for individualized therapy as per the various subgroups based on Ahlqvist etal’s posit. Thus we conclude that the innovative T2D subgroups add to an intriguing model that could stimulate us to get better insight over the pathophysiology of this very wide group of T2D that aids in individualized treatment options on the basis of the underlying etiology of the disease. In these innovative T2D subgroups of adult onset disease that would aid in giving some antidiabetic agents that would prove are more advantageous for certain subgroups, considering the major pathophysiology in addition to avoidance of end organ injury. To start with it is just the initiation of trying to get in individualized therapy for T2D, along with studies that start performing evaluation of the current existence in addition to innovative drugs, prospectively in various subgroups possessing separate metabolic phenotypes to succeed in making therapy more individualized.
Type2 diabetes mellitus; Individualized treatment; Classification of diabetes mellitus; Insulin sensitivity; βcell function; SGLT2 inhibitors; Weight control
Type2 Diabetes mellitus (T2D) represents a global health condition that as per the International Diabetes Federation (IDF) would influence 700 million people by 2045 [1]. A multidisciplinary strategy is needed for its management for avoiding along with reduction of complications. Glucosedecreasing medicines are the crucial agents for regulation of blood glucose amounts. Escalated blood glucose amounts in case of T2D get reasoned out by Insulin Resistance (IR), combined with a decrease in βcells function. In case of certain patients of T2D it is the dominance of IR, while in others decreased insulin liberation is the basic impairment. Lot of modes exist behind βcells failing in addition to reduction of function of insulin sensitivity. Inspite of lot of factors responsible for the etiopathogenesis of the disease there are restricted treatment methods that are usually not individualized in relation to the basic etiology of hyperglycemia. Significantly T2D represents a systemic syndrome influencing practically all the tissues in the body, with the disease being correlated with a lot of diseases that include Cardiovascular Disease (CVD), Kidney disease, Non- Alcoholic Fatty Liver Disease (NAFLD), Alzheimer’s disease, in addition to different cancers. Till date neither of the glucose decreasing-agents has made any main influence on end organ protection. Nevertheless recent studies have demonstrated that Sodium-glucose Cotransporter-2(SGLT2) inhibitors, in addition to Glucagon Like Peptide 1(GLP-1)-1 Receptor Agonist (GLP-1RA), decrease the risk of CVD, illustrating end organ protection, that is further than just glycemic regulation. Here the classification of T2D, brief etiopathogenesis, actions of variety of medication groups on insulin sensitivity along with βcells function, with the objective to give more individualized treatment strategies. Earlier we had reviewed extensively on the etiopathogenesis, management of obesity in addition to its complications like DM, in addition to etiopathogenesis of T1Dalong with their treatment modes in details in addition to their complications like CVD, DN, neuropathy and retinopathy, HF, NAFLD, NASH [2-25]. Here we further considered to present how individualized treatment modalities can be done with the idea of utilization of classification of DM as per the different metabolic phenotypes and decide how we can use individualized treatment modalities for treatment of Type2 Diabetes mellitus (T2D) patients.
Type2 Diabetes mellitus (T2D) represents a disease which implicates a lot of metabolic impairments that possess properties of hyperglycemia that occurs secondary to pancreatic βcells failing in addition to insulin sensitivity reduction. The risk factors for generation of T2D are obesity along with Insulin Resistance (IR). Nevertheless, maximum obese as well as people with IR never generate T2D that gets reasoned by robust genetic constituents correlated with T2D. de Fronzo in 1988 had revealed that generation from dysfunctional glucose tolerance to T2D is basically secondary to reduction of βcells function as well as not associated with changed insulin modulated glucose uptake, while IR usually exists prior to hyperglycemia, with an escalation of HbA1c taking place [26]. Nevertheless, it needs to be understood that therapy of IR would decrease the βcells load in addition to alleviate hyperglycemia. The risk of generation of T2D gets robustly inherited, with detailing of lot of genetic correlations [27]. Maximum of the genetic correlations have been attributed towards βcells function in addition to occasional correlation with IR [27], despite this might be secondary to no correct measures present for us in large cohorts.
In T2D, βcells failure has been correlated with 24%-65%loss of βcell mass, along with a 50% -97% deletion of insulin liberation ability of βcells [28]. Pancreatic βcells, to start with are able to tackle the IR in peripheral tissues by greater generation of insulin, resulting in supraphysiological insulin amounts.
Gradually βcells failure results causing escalated post prandial along with fasting glucose amounts, despite persistent hyperinsulinemia. Modes correlated with βcells failure are IR, glucotoxicity, lipotoxicity, βcells senescence, dedifferentiation, as well as/or apoptosis [29-31]. First degree relatives of patients with type2 Diabetes possess dysfunctional insulin liberation, with lesser regular pulsatile nature of insulin liberation [32].These alterations in insulin pulsatile liberation might result in down regulation of insulin function in addition to points to a crosstalk among dysfunctional βcells action in addition to deterioration of IR [33].Thus it has to be clarified if insulin resistance occurs prior to β cells failure in all the subjects generating T2D.
The other main point in the generation of T2D is the generation of whole body in addition to peripheral Insulin Resistance (IR) that occurs over a point of time slowly. Since skeletal muscle, that represents the biggest organ of body, takes up 85% of the postprandial glucose uptake, skeletal muscle IR, aids in the generation of hyperglycemia [34]. In case of skeletal muscle, the properties of IR are decreased intracellular insulin induced glucose uptake in addition to handling secondary to decreased insulin stimulated GLUT4 getting transferred to the cell membrane followed by glycogen generation subsequently (Figure 1) [35,36].
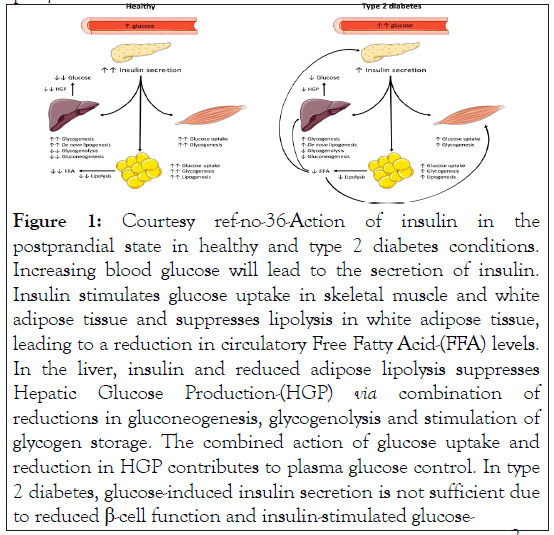
Figure 1: Courtesy ref-no-36-Action of insulin in the postprandial state in healthy and type 2 diabetes conditions. Increasing blood glucose will lead to the secretion of insulin. Insulin stimulates glucose uptake in skeletal muscle and white adipose tissue and suppresses lipolysis in white adipose tissue, leading to a reduction in circulatory Free Fatty Acid-(FFA) levels. In the liver, insulin and reduced adipose lipolysis suppresses Hepatic Glucose Production-(HGP) via combination of reductions in gluconeogenesis, glycogenolysis and stimulation of glycogen storage. The combined action of glucose uptake and reduction in HGP contributes to plasma glucose control. In type 2 diabetes, glucose-induced insulin secretion is not sufficient due to reduced β-cell function and insulin-stimulated glucose-
Besides skeletal muscle, liver IR causes escalated basal Endogenous Glucose Generation (EGP) along with reduction of insulin suppression of EGP, that aids in the greater plasma glucose amounts (Figure 1) [31].
Adipose Tissue (AT) insulin resistance aids in hyperglycemia by glucose uptake reduction, despite AT glucose uptake is usually believed to be comparatively less in humans [37]. Nevertheless, AT IR further results in decreased hampering of lipolysis by insulin that can lead to escalated Free Fatty Acids (FFA) amounts in blood (Figure 1) [38]. Greater circulating FFA can aid in skeletal muscle IR. Moreover greater lipolysis rates further result in greater glycerol that is believed to have a significant part in gluconeogenesis along with EGP [39]. Figure 1 illustrates the postprandial insulin effects in T2D.
uptake in muscle and White Adipose Tissue-(WAT) as well as insulin-stimulated suppression of HGP is blunted. Insulin resistance in WAT leads to blunted suppression of lipolysis by insulin, producing higher FFA levels that subsequently negatively affect skeletal muscle and FFA, HGP.
There exists a robust correlation among type2 Diabetes mellitus in addition to obesity, with about 90% of all T2D patients being overweight or obese. Fat mass expansion makes sure that storage escalated nutrient/energy occurs; nevertheless, when AT expansion capacity becomes restricted or impaired [40], an escalation of circulating FFA in addition to enhancement FFA uptake by liver as well as skeletal muscle can take place, where competition with glucose can cause substrate oxidation, that as per Randle cycle can aid in insulin resistance [41]. Besides that FFA can further collect in non-adipose tissue, along with ectopic fat collection has been demonstrated to be a key factor for the generation of IR in the liver as well as skeletal muscle, basically secondary to meddling by diacylglycerol, in addition to ceramides (of the rest) with the insulin signaling pathway [42]. Further enhancement of FFA uptake is also correlated with Oxidative Stress (OS), inflammation as well as cell demise. Lipotoxicity can take place in a variety of tissues like skeletal muscle, liver, heart, arteries, pancreas that produces separate phenotypes or end organ injury in patients secondary to which organs are implicated maximum. In case of muscle, fat collection intervenes with the insulin initiated GLUT4 getting translocated, whereas in liver Non-Alcoholic Fatty Liver (NAFL) is correlated with hepatic insulin resistance in addition to escalated generation of Very Low Density Lipoprotein (VLDL)- Triglycerides (TG), which aid in the generation of atherosclerotic dyslipidemia [43]. Further hepatic insulin resistance generation can also be secondary to pulsatile insulin getting administered in the hepatic portal vein along with finally in hepatocytes [33]. This posit points that deranged insulin administration as is seen in T2D, might result in impairment of hepatic metabolism or selective IR via FoxO1, aiding in collection of lipids [44]. Selective IR is a pathological condition where insulin doesn’t cause reduction of HGP, yet insulin activation of de-novo lipogenesis through stimulation of SREBP-1c does not get implicated as well as further escalated secondary to correlated hyperinsulinemia resulting in, more fat collection [45]. In case of Pancreas βcells getting exposed to chronic escalated amounts of FFA result in Endoplasmic Reticulum (ER) stress in addition to mitochondrial impairment, that can lead to cell injury as well as ultimate dysfunctional insulin liberation [46].
Chronic hyperglycemia has further been demonstrated to have toxic actions on βcells as well as other tissues, for which the term glucotoxicity was coined. Glucotoxicity aids in βcells failure in addition to decreased insulin sensitivity in the liver through variety of events, like ER stress, mitochondrial impairment, Oxidative stress, along with inflammation [47]. Additionally, with chronic hyperglycemia, glycogen storage occurring in βcells has been, illustrated to be correlated with apoptosis [48]. If Glucotoxicity influences skeletal muscle insulin sensitivity is still debatable and needs more exploration [35].
Besides obesity, age is another factor that decides the generation of T2D that has long been believed to be a disease correlated with exaggerated ageing. Wijsman documented that familial longevity had the properties of escalated insulin sensitivity in contrast to a group possessing similar age, sex in addition to body make up [49]. With age decrement of physical activity along with muscle mass is usually seen, that is factors that directly aid in the generation of skeletal muscle insulin resistance. Additionally, ageing is usually correlated with an escalation of fat mass which might aid in generation of lipotoxicity along with IR. Cellular stress reactions can result in a state where cellular Senescence possessing the properties of cell cycle arrest, apoptosis resistance, in addition to Senescence- Associated Secretory Phenotype (SASP), that has a negative impact on organ functions. That insulin resistance exaggerated βcells Senescence in human islets (Aguayo-Mazzucalo) was demonstrated. Furthermore in mouse models of type1 Diabetes, it got illustrated that deletion of Senescent cells stopped immune modulated βcells break down as well as avoided Diabetes [50]. Hence both enhancement of insulin sensitivity along with escalated apoptosis of senescent islet cells could result in enhancement of βcells functions.
The Baltimore Longitudinal study of ageing demonstrated that a reduction of insulin liberation occurs with age based on Body Mass Index (BMI) in addition to Adipose tissue spread [51].This might reason out why the Prevalence of T2D is correlated with escalated age in the population.
Maximum insulin resistant people do not generate T2D along with genetic constituents might reason out why certain insulin resistant people generate T2D. GWAS (Genome Wide Association Studies) have isolated a SNP which are correlated with function of βcells. Of the certain variants are present over 40 loci as well as can escalate the risk of T2D. Despite greater than 400 gene variants have been correlated with the existence of T2D, the presently isolated variants have only accounted for 10% of genetic influence for the chances of generation of T2D [52]. As compared to that maturity onset Diabetes in the young is monogenic Diabetes, accounting for 2-5% of Diabetes patients [53].
An International work group generated a new Classification which included Type1 Diabetes mellitus (T1D), T2D in addition to Gestational Diabetes Mellitus (GDM) in 1979 [54].They further added an Impaired Tolerance Test (IGT) group: people who did not meet the criteria for DM but had an escalated fasting as well as 2 h postprandial glucose amounts. This Classification got reviewed in 1997 as well as broke in 2 i) Impaired Fasting Glucose (IFG) in addition to ii) IGT [55].
Over 40 year. Subsequent to this Classification system was initially advised, insight into Diabetes pathophysiology has become further complicated. Nevertheless, still just 2 main Classifications T1D as well as T2D. With the challenge now for more individualized medicine approach, a further refinement of Classification system would be aid in generation of innovative drugs that correct the basic aetiology of the syndrome in addition to prescription of the best medicine currently prevalent for avoiding propagation of disease along with end organ injury.
In 2018, Ahlqvist pointed a new Classification system of adult onset Diabetes that at minimum takes into account, the heterogeneous phenotype of T2D [56]. In the sub group Classification, adult onset Diabetes is further sub Classified into 5 sub-groups or clusters with utilization of 6 quite common parameters that can be easily acquired from the clinical scenario; namely i) BMI ii) HbA1c iii) Glutamic Acid Decarboxylase Antibodies (GADA) iv) Homeostasis Model Assessment 2 (HOMA2) to evaluate βcell function (HOMA2B) along with insulin resistance (HOMA2IR) depending on the fasting glucose as well as C peptide amounts. Data driven non supervised cluster evaluation was performed utilizing large Swedish as well as Finnish cohorts that included all new incidents of adult onset Diabetes. Data driven non supervised cluster evaluation made the conclusions that 5 novel sub groups for newly diagnosed adult onset Diabetes depending on the variables defined earlier; i) Severe Autoimmune Diabetes (SAID) ii) Severe Insulin Deficient Diabetes (SIDD) iii) Severe Insulin Resistance Diabetes (SIRD) iv) Mild Obesity related Diabetes (MOD) v) Mild Age-Related Diabetes (MARD) (Figure 2). SIDD in addition to SAID had the properties of earlier onset-Diabetes, having a lesser BMI in relative terms, bad regulation in metabolic terms (greater HbA1c), along with insulin deficiency (based on low HOMA2B index). The variation among SAID in addition to SIDD is the existence of GADA in SAID but not in SIDD. SAID possesses an overlap with T1D as well as Latent Autoimmune Diabetes in Adults (LADA). LADA has genetic properties akin to T1D, but in a clinical scenario, they usually possess characteristics akin to T2D patients, thus usually get diagnosed as T2D. With the application of similar cluster evaluation in an independent German cohort demonstrated that the patients allotted to the SIDD group further revealed signs of autoimmunity [57].
Figure 2: Courtesy ref no-36-Visual representation of the characteristics of the subgroups as suggested by Ahlqvist et al. [56]. Severe Insulin-Deficient Diabetes (SIDD) is characterised by a relatively low age and BMI, a high HbA1c, less marked insulin resistance, but severe β-cell insulin deficiency. Severe Insulin- Resistant Diabetes (SIRD) is characterised by a relatively high age and BMI, a relatively low HbA1c, severe insulin resistance, but no insulin deficiency. Mild Obesity-related Diabetes (MOD) is characterised by a relatively low age at diagnosis, a high BMI, relatively low HbA1c, and mild insulin resistance and insulin deficiency. Mild Age-Related Diabetes (MARD) is characterised by a high age at diagnosis, a relatively low BMI, and mild insulin resistance and insulin deficiency. More severe insulin resistance/ deficiency is indicated with a larger stop sign.
SIRD possesses the properties of a greater BMI (over weight to obese) in addition to severe IR (based on high HOMA-IR index). In SIRD, βcells function is lesser dysfunctional in contrast to SAID in addition to that SIDD (greater HOMA2B index) as well as HbA1c amounts are lesser. Both SIRD in addition to SIDD were earlier diagnosed as T2D although are markedly separate types of robust T2D. MOD along with MARD have the properties of mild insulin deficiency (HOMA2B index greater than SAID in addition to SIDD).The variation among MOD along with MARD is dependent on age at the time of diagnosis as well as BMI; MOD has the properties of greater BMI (obesity), whereas MARD possessing greater age at the time of diagnosis. Hence SAID is made up of patients which are presently diagnosed as T1D or LADA, whereas in the rest 4 clusters get diagnosed as T2D.
The propagation of disease as well as risk of end organ injury appear to be separated by sub groups, SAID in addition to SIDD possessing greater HbA1c at baseline in addition to during follow up in contrast to rest of sub groups also correlated with an escalated risk of ketoacidosis [56,57]. SIRD has a correlation with a great prevalence of Non-Alcoholic Fatty Liver Disease (NAFLD), along with fibrosis at diagnosis [56,57] in addition to Diabetic Kidney Disease (DKD) as well as End Stage Renal Disease (ESRD) [56], but on rectification for baseline Kidney function, no variation among separate sub groups [58]. That is patients with SIRD generate end organ injury before they get diagnosed with Diabetes. Conversely neuropathy as well as retinopathy are more commonly correlated with the SIDD group [56,57]. The sub groups also vary by the early treatment prescribed in the cohort at the time of diagnosis. As far as the SAID group is concerned 42%-76% are receiving insulin therapy as well as 29%-44% of SIDD patients were receiving insulin therapy [56,57].
Despite T2D being a heterogeneous syndrome as pointed by huge inter person variations with regards to insulin resistance, βcells function, along with autoimmunity, the present treatment approaches basically concentrate on reduction of glucose in addition to HbA1c for avoidance of end organ injury. Since Atherosclerotic Cardiovascular Disease (ASCVD) still continues to be the commonest cause of morbidity as well as mortality in T2D patients, the guidelines are very implicit with regards the degree to which the various medicines have exhibited reduction in risk of CV processes. Other end organ diseases correlated with T2D like Chronic Kidney Disease(CKD), NAFLD, neuropathy as well as retinopathy are further more significant to take into account when decision of proper treatment for patients with T2D are decided. Nevertheless, currently, anticipation of disease propagation or risk of end organ injury in persons with T2D is not fully clear. Thus for it to be more efficacious in addition to cost beneficial it would be more advantageous if more precise ways of anticipating risks for treatment of patients with greater aggression, in those that possess a greater risk in contrast to those with lesser risk.
Subsequent to initial guidelines with regards to lifestyle modifications, weight reduction, along with escalated physical activity, patients current situation remain the reference points for the choice of an antihyperglycemic agent. Thus the guidelines mostly take into account patients chances of generation of CV processes, weight as well as chances of hypoglycemia when trying to select the antihyperglycemic agent.
Other factors on which decision is based remain the cost of medicine in addition to possessing proven effectiveness. Hence the latest guidelines of the American Diabetes Association (ADA) along with European Association For the Study of Diabetes (EASD) of 2020, metformin still occupies the first line therapy having the knowledge of its specific profiles when evaluation for cost benefit along with tolerance [59,60]. The mode of action of metformin on glucose regulation has not been totally worked out with both liver as well as intestine having been pointed as the major target tissues, though its mechanistic role has been extensively reviewed [61]. Yet, large proportion of patients won’t have the capacity to reach the treatment targets by consumption of metformin alone, thus ultimately need the adding of a second line therapy.
Second line therapy choice will be based on the patients having generated ASCVD, CKD, or Heart Failure (HF). In case still these have not developed as yet, one makes the opinion that is dependent on the risk of side effects like hypoglycemia, weight gain, cost as well as what patients choice is. Nevertheless, little proof exists to be able to guide the 2nd line therapy or for that matter even 3rd line for attaining glucose homeostasis.
The novel Classification system for Diabetes pointed by Ahlqvist demonstrated the heterogeneity of T2D, concentrating on various factors that are IR, βcell impairment [56]. This new Classification can aid in generation of new, more individualized strategy by evaluation of the association with antihyperglycemic agent as well as their actions on the mode of action of T2D etiology. With this Classification, it might also be that patients at greater risk receive more combative treatment at diagnosis for avoidance of end organ diseases correlated with the sub groups of Diabetes. Currently 5 separate classes of 2nd line antihyperglycemic agents that have been advocated by ADA in addition to EASD, DPP-4 inhibitors, glucagon like peptide 1(GLP-1)-RA, Sodium-Glucose Cotransporter 2 (SGLT2) inhibitors, Sulfonylureas, thiazolidinediones. These medicines have been used with success at commercial in view of their capacity to enhance glucose homeostasis, cause reduction of HbA1c, nevertheless possess different in addition to inadequately found modes of action for enhancement of glucose homeostasis in separate methods. This offers a way out for utilization of individualized therapy approach. Thus idea of review is to get insight into the posited working mode of the presently written medicine treatment strategy for the 2nd line antihyperglycemic agents along with the data present on the actions of these treatment agents on βcells function along with insulin sensitivity.
There has been a suggestion that probable treatment approaches for the novel SIDD, SIRD, MOD along with MARD sub groups that at present are made up of the largest sub groups of T2D, since these groups might improve from separate medicines. Significantly, very minimal results are available for making the correct decision for the patients based on their metabolic phenotypes. Thus these are certain posit generating suggestions although can’t be considered in the form of recommendations. For SAID groups not detailed as this entails T1D as well as LADA, which is a heterogeneous group needing insulin treatment. Sulphonylureas also not detailed as their actions on βcells function along with insulin sensitivity are well developed. Sulphonylureas do not possess any action on insulin sensitivity, but to start with will enhance insulin function. Subsequent to 1-2 years of treatment HbA1c amounts will escalate, pointing to a deterioration of βcells function [62]. Nevertheless, it needs to be appreciated that Sulphonylureas have been demonstrated in cases of MARD [58], illustrating that Sulphonylureas still have a place in long term therapy of this T2D subgroup. Insulin also not detailed-extensively reviewed, with guidelines on when to as well as under which situations insulin is beneficial over rest of the second line therapy agents [59]. Medicines which cause enhancement of glucose uptake amounts including insulin treatment, aid the rest of βcells by compensation for insulin needs by rectification of hyperglycemia. The beta cell rest is too detailed to be discussed here, but it suffices to say that at present no clinical proof that any medicines change the propagation of Disease with regards to beta cell function enhancement but for acute actions [63]. Nevertheless, it needs to be addressed that utilization of insulin in T2D patients possesses certain disadvantages like the chances of enhancing the risk of weight escalation, that might result in IR, in addition to treatment with insulin in T2D patients might enhance the risk of CVS complications [64].
Here we consider Second line therapy for T2D patients on basis of specifically utilization of human trials utilizing hyperinsulinemic clamps or mixed meal tests, if feasible, since these approaches are considered the gold standard ways for evaluation of beta cell function along with insulin sensitivity.
Sodium-Glucose Cotransporter 2(SGLT2 inhibitors)
SGLT2 inhibitors represent an innovative kind of agents resulting in glucose reduction by action on SGLT2 that gets expressed in the first segment of the proximal tubules in the Kidneys. SGLT2 results in about 90% reabsorption of glucose from the Kidneys. Inhibition of SGLT2 causes urinary excretion of 60-80 g glucose daily, with the precise amounts based on the plasma glucose amounts in addition to enhanced Glomerular Filtration Rate (GFR), resulting in decrease of HbA1c of 0.6%-0.9% as well as FBG by 1.1-1.9 mmol/l in contrast to placebo [65].The mode by which SGLT2 inhibitors cause glucose reduction is quite simple as well as direct, by enhancement of urinary loss of glucose, a mode that is independent of insulin action [65]. Both glucose along with energy elimination initiates adaptive reactions which might aid in the advantageous actions on of these agents. SGLT2 inhibitors are correlated with body weight reduction [65], lesser Blood Pressure (BP), in addition to positive outcomes of CV death, HF along with propagation of CKD [52-54,rev by us 23-25CV,HF].
SGLT2 inhibitors in addition to beta cell function:
Glucose deletion via urine might enhance beta cell function through reduction in glucotoxicity along with decrease in escalated insulin liberation secondary to reduction in glucose amounts [67]. Despite SGLT2 inhibitors can’t target the beta cells by direct action; their actions on beta cell function have been extensively evaluated in variety of human intervention studies. Both Al-Jobori in addition to Merovci documented a 2 fold enhancement of beta cell function that is determined in the form of escalated insulin liberation/ insulin resistance index (also Known as the deposition index); i.e. the alteration of C peptide amounts divided by the alteration of glucose amounts (Δ C peptide /Δ glucose) divided by insulin resistance) following 2 wk of SGLT2 inhibitors treatment in T2D patients [68,69]. Akin to that Forst demonstrated 2 independent studies of escalated beta cell function as evaluated by enhancement of area under curve for insulin, C peptide/pro insulin ratio at the time of hyperglycemic clamp following 30 days of treatment with SGLT2 inhibitors in T2D patients receiving co treatment with metformin [70].
Various studies illustrated that treatment with utilization of SGLT2 inhibitors enhances beta cell glucose sensitivity. Ferranini documented that 25% enhancement in beta cell glucose sensitivity following just 48h of SGLT2 treatment in patients with T2D in treatment-nave along with and metformin pretreated [71]. Subsequent to 14 days of therapy, escalated beta cell glucose sensitivity were maintained. Three separate studies demonstrated in patients with T2D in treatment-nave or received diet advice, metformin, Sulphonylureas, or a combination of metformin, as well as Sulphonylureas documented that beta cell glucose sensitivity escalated following 48h along with 14days of SGLT2 treatment [68,72].
The propagation of Diabetes is basically secondary to reduction in beta cell function. This implies that long term actions of enhancement of beta cell function can get observed since no propagation in the worsening of HbA1c amounts. The actions of SGLT2 inhibitors were proven in a meta-analysis that included 38 studies of ≥24weeks period that was performed by Zaccardi. On average they revealed an HbA1c decrease of 0.6%-0.9% [65].On concentration on studies possessing a long period (≥104wks) with a measurement of HbA1c SGLT2 inhibition generated a maintained decrease of 0.30%-1.22% [73].
SGLT2 inhibitors along with insulin sensitivity:
Inhibition of SGLT2 can result in enhancement of insulin sensitivity through a decrease of plasma glucose in addition to decreased weight of 1.5-2 kg has been illustrated in patients on SGLT2 inhibitors [65,74]. In the following detailing, glucose reduction through urine might result in activation of lipid oxidation for compensation in humans that could influence the arrangement of escalated fat mass in addition to reduction of ectopic fat stores that have a robust association with the generation of IR [67].
Various studies evaluated the actions of Inhibition of SGLT2 on peripheral insulin sensitivity [71,75,76]. Ferranini documented a reduction in total glucose disposal that was rectified for urinary glucose excretion following acute SGLT2 inhibitors delivery that got maintained following 14d of treatment of patients with T2D that were treatment-naïve or received metformin [71]. Nevertheless, although the reduction in glucose disposal mainly secondary to non-oxidative glucose disposal, peripheral insulin sensitivity, that was evaluated by the ratio of the glucose metabolic clearance rate to the mean plasma glucose amounts at the time of a mixed meal test enhanced markedly following acute delivery, but the escalation did not achieve statistical significance following 14 d of therapy. Merovci observed akin outcomes with the utilization of hyperinsulinemic euhyperglycaemic clamps for evaluation of insulin sensitivity [75]. 14 days of SGLT2 Inhibitors delivery escalated insulin modulated whole body glucose disposal rectified for urinary glucose elimination from 4.3 ± 0.4 to 5.0 ± 0.4 mg/kg/min, that was a significant enhancement in contrast to baseline as well as placebo (4.0 ± 0.5 to 4.3 ± 0.64 mg/kg/min) in patients with type 2 Diabetes mellitus treated with metformin or combination of metformin along with sulfonylureas. Akin to that following 12 wks of a SGLT2 inhibitor treatment, peripheral insulin sensitivity estimated at the time of hyperinsulinemic euhyperglycaemic clamps enhanced in contrast to placeboin patients with T2D that received co treatment with metformin or combination of metformin or an insulin secretagogue [76]. Outcomes akin to this were observed in rest of studies. Hence in patients with T2D that received co treatment with metformin, sulfonylureas, Dipeptidyl Peptidase-IV Inhibitorson (DPPIV) inhibitors or combination of metformin with sulfonylureas, peripheral insulin sensitivity escalated by about 16%-36% in contrast to baseline as well as placebo following SGLT2 inhibitor delivery [69,77]. Conversely Latva Rasku did not observe any enhancement following 8 wks of a SGLT2 inhibitor treatment on insulin sensitivity (estimated in the form of whole body insulin activated M Values) or skeletal muscle glucose uptake in patients with T2D that received co treatment with metformin or combination of metformin with DPPIV inhibitors [78]. Latva Rasku pointed that robust insulin resistance in the patients taking part might reason out why a relatively lesser insulin infusion rate (40 Mu/m2/min) could not pick up an alteration in M Values) [78]. Despite significant reduction in liver fat amounts occurred (proton density fat fraction: 3.7%) this decrease in hepatic fat did not enhance insulin sensitivity (as estimated in the form of repression of EGP) or escalated glucose uptake by the liver.
On the other hand variation of studies documented an enhancement of EGP following SGLT2 inhibitor treatment [71,75-77,79]. The hepatic in addition to probably renal glucose generation makes a compensation for about 50% of glucose eliminated in urine in patients of T2D, thus blunting the reduction in plasma glucose amounts [75]. The precise mode resulting in this compensatory enhancement of EGP is not well understood. It has been pointed that reduction in insulin: glucagon ratioor ANS-modulated mode might be implicated. Alatrach documented that insulin along with glucagon amounts under glucose clamp situations (avoidance of a reduction in glucose amounts) did not vary among subjects receiving SGLT2 inhibitors or placebo [80]. This is against the significant part of the insulin: glucagon ratio in modulation the escalation of EGP following SGLT2 inhibitor hampering. Solls-Herrera along with Daniele posited that renal Autonomic Nervous System (ANS) afferents have a significance for enhancement of EGP following SGLT2 inhibition [81,82]. They evaluated the actions of SGLT2 inhibition on EGP in Kidney transplant patients with either both residual native Kidneys in place or a bilateral nephrectomy. An enhancement of EGP following SGLT2 inhibitor delivery took place in both groups. Whereas the enhancement of EGP in their native Kidneys could be compared by other studies, the enhancement of EGP got blunted in those patients with a bilateral nephrectomy. This observation pointed that the part of Kidneys as well as /or ANS in EGP following SGLT2 inhibition continues to be not clear.
SGLT2 inhibition has been demonstrated to alter substrate oxidation that might be beneficial with regards to insulin sensitivity. Hence a reduction in glucose Oxidation along with enhancement of lipid oxidation in addition to ketone generation has got documented that could aid in enhancement of beta cell function along with insulin sensitivity by decreasing ectopic fat as well as mitigation of lipotoxicity [77,83]. Nevertheless, escalated fatty acids Oxidation is correlated with enhanced adipose tissue lipolysis along with escalated fatty acids flux which would result in reduction of glucose uptake in skeletal muscle, resulting in reduced skeletal muscle insulin modulated glucose uptake in skeletal muscle. Nevertheless, minimal insight is there with regards to alteration in potentially deleterious intracellular lipids, with the maximum proof that ectopic fat reduction in liver [78,84],visceral fat[85], epicardial fat[86], subsequent to treatment with SGLT2 inhibitor.
Thus concluding that SGLT2 inhibitors in a modest, albeit significant enhancement of beta cell function in addition to beta cell glucose sensitivity. Long time studies pointed that maintenance of glucose reducing action following a minimum of 2 yr. of therapy. No washout studies have got performed as far as we know to evaluate if enhancement of beta cell function gets maintained following omitting of SGLT2 inhibitors. As far as insulin sensitivity is concerned, various research groups have documented escalated insulin sensitivity, but the degree of enhancement was not much. It is posited that advantageous actions of SGLT2 inhibitors therapy is basically secondary to reduction in glucotoxicity. Nevertheless, Clinical trials evaluating along with insulin sensitivity over longer time duration are minimal. It is possible that treatment for over 3-4 months might demonstrate separate outcome. Like data point that following 3-4 months, energy decreases get compensated by escalated food intake which could reason out why body weight reduction doesn’t occur following this period of time [87].The results present on beta cell function along with insulin sensitivity in addition to the knowledge that SGLT2 inhibitors act independent of insulin pointed that SGLT2 inhibitor therapy might be advantageous in all 4 posited novel subgroup of T2D. The first study to evaluate the effectiveness of SGLT2 inhibitors in addition to glucagon like peptide receptor agonists in patients with SIDD as well as SIRD has initiated enrollment (Clinical trials.govIdentifier:NCT04451837).
Glucagon like peptide1 receptor agonists
Glucagon Like Peptide (GLP-1) represents a hormone, generated by the L cells of the intestine in reaction to food consumption, specifically in meals possessing a great amount of fat along with carbohydrate. GLP-1 delivery enhances glucose amounts via separate modes that include glucose based insulin liberation, decrease food consumption, reduction in body weight in addition to decreased amounts of glucagon. Glucagon like peptide1 receptor agonists (GLP-1RA) decrease HbA1c by a range varying from 0.5%-1.5% [88].
GLP-1RA along with beta cell function: Of the anticipated mechanistic modes of GLP-1RA is through a direct effect on βcells. Bcells themselves show expression of GLP-1 receptors. GLP-1 receptors belong to G Protein Coupled Receptor (GPCR), with their activation causing an enhancement of cAMP in addition to Protein Kinase A (PKA) action that facilitates insulin liberation from βcells [89]. The LIBRA trial evaluated beta cell function in patients in whom type2 Diabetes mellitus diagnosis had been made recently, who received insulin treatment for 4 weeks prior to getting randomized with either a GLP-1RA or placebo for 48 weeks along with observed that enhancement of beta cell function occurred as estimated by insulin liberation sensitivity index 2 in the active group [90]. In another randomized controlled trial performed in patients with T2D in contrast to actions of a short acting GLP-1RA vs. placebo for 3yrs as well as found beta cell function escalated as estimated by the Mari model, an approach that evaluates beta cell function from results received at the time of an Oral Glucose Tolerance Test (OGTT) [91].
Anholm observed that 12 weeks of metformin with a GLP-1RA resulted in a significant enhancement of beta cell function as evaluated by the disposition index in contrast to a metformin or placebo group in a randomized, double blind crossover trial [92]. An additional randomized controlled trial GLP-1RA with metformin or metformin along with lifestyle interventions on beta cell function in patients in whom type2 Diabetes mellitus diagnosis had been made recently, it was observed that Liraglutide escalated beta cell function that was expressed, in the form of beta cell insulin liberation at the time of an OGTT in contrast to a control group within a 15months time duration [93]. The positive action of both short as well as long acting GLP-1RA on beta cell function has been illustrated in variety of randomized Clinical trials.
In animal models of Diabetes, it has been documented that treatment with GLP-1RA escalates the beta cell function, basically via proliferation as well as differentiation [94]. Nevertheless, if GLP-1RA escalates the functional beta cell mass in human beings in not known as yet. The outcome of washout studies illustrated no long lasting actions on the beta cell function, hence pointed that no action on functional beta cell mass in addition to the actions observed on beta cell function appeared to be acute [90,91].
GLP-1RA as well as insulin sensitivity: The acute actions of short acting GLP-1RA was evaluated by Gastaldelli on the hepatic in addition to AT insulin sensitivity that was determined in the form of glucose as well as glycerol tracer kinetics following a 13 C enriched glucose load [95]. This study was performed in patients with T2D along with persons having IGT. They observed that acute treatment with GLP-1RA escalated hepatic in addition to AT insulin sensitivity in contrast to placebo. The continued action of GLP-1RA on insulin sensitivity was evaluated by Zander [96]. They examined the actions of continued/confusion of GLP-1RA vs. saline infusion with the utilization of a portable pump for 6 weeks in patients withT2D as well as observed that insulin sensitivity as estimated by hyperglycemic euglycemic clamps enhanced by 77%. Nevertheless, this action on insulin sensitivity might have been over determined since the study did not get randomized or blinded. The enhancement of insulin sensitivity was correlated with a reduction in fasting plasma glucose along with FFA amounts that could have aided in this action. The action of GLP-1RA as well as metformin vis a placebo by Anholm [97], on insulin sensitivity in obese as well as overweight patients that presented with newly diagnosed type2 Diabetes and coronary artery disease. Evaluation of insulin sensitivity was performed with the utilization of ISI composite, an estimation of whole body insulin sensitivity, derived from a formula which combines results derived from OGTT in addition to results derived from fasting plasma glucose as well as insulin [98]. GLP-1RA as well as metformin escalated beta cell function as determined by the disposition index by 40% in contrast to metformin as well as placebo; nevertheless insulin sensitivity was not significantly separate among the groups [97].
The actions of GLP-1RA on hepatic insulin sensitivity was analyzed by Armstrong, as estimated by repression of EGP, following 12 weeks of GLP-1RA therapy vs. placebo in individuals with Non-Alcoholic Steatohepatitis (NASH) [99]. A hyperglycemic euglycemic clamps utilization was done prior to as well as following treatment, it was observed that GLP-1RA caused reduction of EGP in contrast to placebo (-9.3% vs. -2.5%). GLP-1RA further caused significant reduction of body weight in the intervention group in contrast to placebo. The actions of GLP-1RAon hepatic fat amounts were as estimated by Magnetic Resonance Spectroscopy (MRS) by Dutour, in obese patients with T2D [100]. Subsequent to 26 weeks of therapy, they observed a significant decrease in hepatic fat amounts in the intervention group in contrast to placebo (-23.8% vs. +12.5%). This decrease in fat in the liver had a greater association with body weight reduction.
Actually the actions of GLP-1RA on body weight might offer the reason for the advantageous actions on liver as well as peripheral insulin sensitivity which have been seen. A meta-analysis which included 25 trials for contrasting GLP-1RA with placebo, insulin or other glucose suppressing agents observed that GLP-1RA resulted in a significant decrease in body weight [101]. The outcomes documented a mean variation of-2.9kg body weight reduction in the intervention group in contrast to a control group. Akin to that Davies documented the long term actions on body weight following 56 weeks of therapy in contrast to placebo in overweight as well as obese individuals with T2D in addition to documented significantly greater body weight reduction in the intervention group in contrast to a placebo group [102]. Other probable reasons for the action on insulin sensitivity might be a correlation that has been observed in animal models among GLP-1RA treatment along with reduction in inflammation [103]. Lynchetal on evaluation of the correlation among GLP-1RA treatment as well as invariant Natural Killer T (NKT) Cells in human along with mice AT, he found that GLP-1RA resulted in activation of iNKT Cells [104].
Intriguingly, activation of iNKT Cells can result in decrease in body weight. Hence GLP-1RA may results in partial weight reduction in addition to cause enhancement of insulin sensitivity by action on the immune system.
Thus concluding that GLP-1RA escalates beta cell function at the time of treatment, although this action does not last following omitting these agents [105]. The action of GLP-1RA treatment on glucose regulation appears to majorly depend on the capacity to escalate insulin liberation with the aid of enhancement of insulin sensitivity through weight reduction in addition to immunomodulation actions. Nevertheless, a restricted knowledge on alterations in insulin sensitivity following GLP-1RA delivery exists.
Present guidelines prove that GLP-1RA is a Second line therapy in obese patients having a diagnosis of Cardiovascular Disease (CVD). It is pointed by Veelen [36], that GLP-1RA treatment might further be the treatment of choice for the obese subgroups that were detailed by Ahlqvist [56] that includes SIRD, MOD in addition to SIDD. In view of the nausea to start with GLP-1RA might not be that advantageous for the MARD group, in view of age of onset along with lesser risk of Diabetescorrelated end organ injury.
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors represent a class of glucose reducing agents which hamper the enzyme DPP-4. Expression of this enzyme occurs on the cell surface like adipocytes, liver, Kidney along with small intestine in addition to glucose reduction they cause reduction of peptide activities, like that of GLP-1, Glucose dependent Insulinotropic Polypeptide (GIP). DPP-4 Inhibitors possess the characteristics of competitively hampering, besides a great affinity towards DPP-4. DPP-4 Inhibitors decrease HbA1c varying from a range of 0.5%-1% [106].
Beta cell function as well as DPP-4 Inhibitors: The actions of DPP-4 Inhibitors on glucose metabolism is believed to be basically by the enhancement of incretion accessibility like GLP-1 as well as GIP, that are implicated for escalated insulin liberation along with reduction of glucagon liberation following a meal [107].The action on beta cell function got proven in prior studies. In a meta-analysis that included 23 a randomized, placebo controlled studies correlated DPP-4 Inhibitor treatment with a significant enhancement in HOMA-B, in contrast to placebo [108]. On utilization of DPP-4 Inhibitors as add on treatment, a significant enhancement in HOMA-B was observed. HOMA-B, basically estimates the insulin liberation, with only limited studies having estimated the action of DPP-4 Inhibitors on beta cell function with the utilization of golden standard approaches.
In case of animal models of obesity, therapy with DPP-4 Inhibitors for 11 months had a greater correlation with Beta cell function, as estimated by the oral disposition index, received at the time of an OGTT, but not correlated with an escalation of βcell mass in contrast to controls [109]. In human beings on evaluation of the actions of DPP-4 Inhibitors along with metformin in contrast to metformin along with placebo, on the liberation ability of βcells, Derosa, illustrated that by utilization of euglycaemic-hyperinsulinemic as well as hyperglycaemic clamp in combination with following arginine activation, they observed escalated Beta cell function, which when it was expressed in the form of disposition index following 12 months of DPP-4 Inhibitor treatment (from 163.8 ± 37.9 to 214.2 ± 48.4 nmol/lx/ μmol/kg) in contrast to controls (from 163.6 ± 37.7 to 279.5 ± 56.9 nmol/lx/μmol/kg) [110].
Despite the actions of DPP-4 Inhibitors are basically believed to be through enhancement of incretin amounts, Aulinger [111], evaluated the actions of DPP-4 Inhibitors on glucose homeostasis in patients with T2D following blockade of GLP-1 action via utilization of GLP-1 Receptor antagonist. Intriguingly, they observed significant actions of DPP-4 Inhibitors on insulin liberation at the time of an OGTT inspite of GLP-1 Receptor blockade. In non-diabetic individuals Yanagimachi [112], determined the incretin amounts, following DPP-4 Inhibitors delivery at the time of an OGTT and they observed DPP-4 Inhibitors delivery, besides resulting in enhancement of GLP-1, further escalated the amounts of bioactive GIP.
Insulin sensitivity as well as DPP-4 Inhibitors: The actions of DPP-4 Inhibitors on insulin sensitivity have got evaluated in animal models. Like Pospisiliketal [113], observed an enhancement in insulin modulated glucose uptake in muscle tissue along with escalated insulin sensitivity as estimated through the Matsuda index following treatment with DPP-4 Inhibitors in contrast to controls. However, in case of human beings, the actions of DPP-4 Inhibitors on insulin sensitivity continue to be debatable. The actions of DPP-4 Inhibitors in the form of add on treatment on insulin sensitivity in T2D individuals, got evaluated by Derosa, where they observed that following 12, 18 as well as 24 months of therapy in the treatment group in contrast to control group, and significantly reduced HOMA-IR [114]. Nevertheless, HOMA-IR, does not precisely determine insulin sensitivity in case of studies that are interventional. No action of DPP-4 Inhibitors therapy for 6month was observed by Parthan, in contrast to placebo on insulin sensitivity as determined by hyperinsulinemiceuglycaemic clamp in well regulated T2D individuals [115]. These outcomes pointed that, inspite of a drop in HbA1c along with fasting plasma glucose amounts, there appears to an absence of actions of DPP-4 Inhibitors therapy on insulin sensitivity that is in contrast with the actions of GLP-1RA treatment. The probable reason might be that DPP-4 Inhibitors in various studies did not appear to possess any significant actions on weight reduction [108,116].
Intriguingly in animal models of obesity, enhancement of weight has been correlated with escalated DPP-4 expression in hepatic tissues [117]. In case of human beings action of DPP-4 has been correlated with a greater BMI, escalated fat proportion along with NAFLD [118]. These observations might point that DPP-4 Inhibition might be a target to decrease hepatic fat amounts. Actually DPP-4 Inhibitors treatments in animal models has not been correlated with benefits in hepatic steatosis [119] as well as liver fibrosis [120]. Nevertheless, in case of human beings DPP-4 Inhibitors treatments have not proved to be of benefit in NAFLD [121].
Thus concluding that DPP-4 Inhibitors possess a significant action on insulin liberation in contrast to placebo, with possibly there major actions on glucose regulation is through enhancement of insulin liberation instead of possessing an actions on insulin sensitivity. DPP-4 Inhibitors in contrast to GLP-1RA treatment appear to possess no actions on body weight, hence might be less advantageous for patients for whom weight reduction causing agents might prove to be most favourable. Veelen pointed that DPP-4 Inhibitors treatments might be the agents to or preferred in case of SIDD, MARD in view of the absence of DPP-4 Inhibition on body weight along with Insulin Resistance (IR).
Thiazolidenedione
Thiazolidenediones alias glitazones are insulin sensitizers. They got initially invented by screening for hypoglycemic action in ob/ ob mice [122]. Later it was invented that thiazolidenediones escalated insulin sensitivity in animals that showed insulin resistance. In case of human beings, akin outcomes were illustrated, since delivery of thiazolidenediones, led to glucose reduction, in addition to insulin amounts, besides resulting in enhancement of IR along with lipid metabolism. Usually it is agreed upon that thiazolidenediones work in the form of nuclear Peroxisome Proliferator Activated Receptor (PPAR) agonist particularly gamma subtype (PPARγ),which is mainly expressed in White Adipose Tissue (WAT), although in lesser amounts in the muscle, liver as well as heart [123]. On activation of the PPARγ transcription of the PPARγ target genes, which are basically implicated in lipid in addition to carbohydrate metabolism along with immune functions [124]. In view of robust side effects, most kinds of thiazolidenediones that include troglitazone, as well as rosiglitazone got removed from market. At present only Pioglitazone has the approval of European Medical Agency (EMA) along with the USFDA for treatment of T2D. Generally pioglitazone delivery is correlated with plasma glucose decrease of 1.2-2.0 mmol/l, HbA1c reduction of 0.9%-1.3%, in addition to enhancement of body weight of 3,6 kg[125].
Beta cell function as well as Thiazolidenedione: The actions of Pioglitazone on beta cell function got proved in a meta-analysis [126]. With the utilization of monotherapy HOMA-B, escalated by 16% in contrast to the baseline. On combination of Pioglitazone with metformin or Sitagliptin, a little albeit significant enhancement of 9.8 as well as 11.8% in HOMA-B respectively was found in T2D patients. Nevertheless, despite HOMA-B yields certain knowledge with regards to actions of Pioglitazone on beta cell function, trials where utilization of the gold standard for evaluation of beta cell function, the disposition index, are restricted. As far as we know just 2 trials disposition index demonstrated the beta cell function in T2D patients. Gastaldelli [127], along with Tripathy [128], documented enhancement of beta cell function with the utilization of disposition index following Pioglitazone delivery for 4 as well as 6 months, respectively. It is not known the method by which pioglitazone causes enhancement of beta cell function, but there might be direct (expression of PPARγ in Pancreatic islet cells) [129] or in direct actions associated with significant enhancement of insulin sensitivity by pioglitazone.
During longer time duration as determined by the PRO active trial with a mean follow up of 34.5 months, pioglitazone proved to be more efficacious in resulting in HbA1c amounts reduction in contrast to placebo in case of patients who got treatment with metformin or sulfonylurea. The HbA1c amounts reduction occurred at a fast pace as well as remained maintained over the total time duration [130], pointing that the longer time duration of pioglitazone action on beta cell function conservation.
Insulin sensitivity as well as Thiazolidenedione: The actions of thiazolidenediones, on insulin sensitivity in case of human beings has been exhaustively evaluated. Natali as well as Ferranini [131] conducted a systematic review marked 23 papers which determined the actions of thiazolidenediones, on peripheral glucose disposal by utilization of hyperinsulinemic clamps as well as/or EGP with the utilization of glucose tracer evaluation in T2D patients. On combination of data evaluation there was documentation of enhancement in range variation of 31-36% along with 19-33% in peripheral in addition to hepatic insulin sensitivity, respectively, following thiazolidenediones delivery in contrast to baseline or placebo. Nevertheless, in this systematic review, besides inclusion of pioglitazone, troglitazone, as well as rosiglitazone were included. As far as pioglitazone is concerned, various research groups illustrated a statistically significant enhancement inperipheral [131-133], hepatic [127,134], along with AT insulin sensitivity [134,135] in T2D patients.
Since PPARγ is mainly expressed in AT, it is pointed that enhancement in peripheral in addition to hepatic insulin sensitivity, along with beta cell function are indirect as well as predominantly evoked by a reduction in fatty acids efflux from adipose tissue, that escalates insulin modulated glucose uptake as well as decreasing lipotoxicity. That pioglitazone induced PPARγ activation, results in reduction of plasma amounts of triglycerides as well as FFA, is well understood [136]. In view of greater FFA amounts are correlated with ectopic fat collection in addition to insulin resistance, reduction of FFA amounts carries a significant part in enhancement of insulin sensitivity. Actually pioglitazone delivery is correlated with rearrangement of adipose tissue that causes a reduction in ectopic as well as lipid collection, but escalated subcutaneous AT. Promrat were the 1st group that documented the actions of Pioglitazone delivery on hepatic lipid amounts in non-diabetic patients with non-alcoholic steatohepatitis [137]. In this particular trial, significant reduction of the hepatic lipid amounts was demonstrated, from 47.5% to 22.8% following 48 weeks of Pioglitazone delivery, nevertheless the total body fat percentage escalated from 35.8%to 37.6%. The insulin sensitivity index as evaluated by a repetitively sampled iv GTT escalated. The actions of pioglitazone vs. metformin delivery for 10 weeks on insulin sensitivity along with Intramyocellular Lipid amounts (IMCL) in patients with IGT were performed by Rasouli [133]. They documented a significant reduction of the IMCL following pioglitazone in contrast to metformin as well as baseline. The reduction of the IMCL amounts was associated with an enhancement in insulin sensitivity, as estimated through an iv Glucose Tolerance Test (GTT), with a rearrangement of visceral fat towards s/c fat stores. Akin outcomes were documented later in patients with pre Diabetes as well as T2D that were simultaneously treated with diet advice, hypocaloric diets, metformin or insulin resulted in a reduction of the hepatic lipid amounts [134,138,139], IMCL [138], as well as myocardial [139] lipid amounts, in addition to escalated s/c fat [134,138,139]. Although a reduction in ectopc fat occurs, treatment results in escalated body weight, secondary to greater caloric consumption in the patients treated with pioglitazone [140].
Notably all studies were consistent on the pioglitazone’s action on metabolic adaptations. Phielixetal [135], documented enhancement in AT insulin sensitivity but didn’t observe enhancement in peripheral or hepatic insulin sensitivity inspite of a reduction in hepatic lipid amounts following 12 weeks of pioglitazone’s treatment in non-obese patients with T2D. Van der Meer [141], documented reduction in hepatic lipid amounts yet no alterations in intra myocardial lipid amounts or myocardial FA oxidation following 24 weeks of pioglitazone delivery in patients with T2D [141]. Bajpeyi documented a significant switch from IMCL towards Extramyocellular Lipid (ECML) in the gastrocnemius, tibialis anterior as well as soleus muscle‘s with a chances towards a reduction in hepatic lipid amounts following 12 weeks of pioglitazone delivery in patients with T2D [132].These alterations were associated with an enhancement in peripheral insulin sensitivity muscle metabolic flexibity (Δrespiratory quotient) estimated at the time of insulin infusion (80 mu/min/m ) in contrast to the fasted state of a of hyperinsulinemic-euglycaemic-clamp. Substrate oxidation during fasting in addition to mitochondrial function that was evaluated in the form of resting ATP turnover along with the maximum ATP generation rate by 31P-MRS was not influenced by pioglitazone.
Hence concluding, that pioglitazone is efficacious in resulting in reduction of peripheral, AT in addition to hepatic insulin resistance basically via mitigation of lipotoxicity by decreasing ectopic lipid getting stored. Further pioglitazone also manages to induce HbA1c reduction over long duration of time that points that there is enhancement in beta cell function. Nevertheless, these actions do not remain maintained following pioglitazone omission [142]. Pioglitazone might work to be a robust treatment for a restricted group of patients in whom overcoming IR in addition to NAFLD treatment are much more significant in contrast to the side effects of weight gain, osteoporosis [143] along with water retention, escalated risk of Heart Failure (HF) [144].Thus it was posited by Veelen, that pioglitazone might be advantageous treatment for SIDD as well as SIRD, in addition to it needs to be prevented in MOD as well as MARD in view of adverse actions [36].
Type2 Diabetes mellitus represents a heterogeneous disease, possessing a complicated metabolic disturbances resulting in hyperglycemia in addition to beta cell function Impairment. Various second line therapy choices are present currently; nevertheless, selecting the maximum appropriate choice of antidiabetic agents might be a tough job. The classification system provided by Ahlqvist yields a broad spectrum of the disease which aids in getting greater insight in the basic metabolic etiology of T2D.
Depending on the documented actions of the presently existing antidiabetic agents on beta cell function, insulin sensitivity along with metabolism, certain medicines might be more appropriate for the treatment of a subgroup of patients with T2D. Metformin continues to be the first-line therapy for glucose regulation of patients with T2D along with probably works as a first-line therapy for patients in all 4 T2D subgroups. Metformin might prove to be enough in form of monotherapy in milder disease that basically includes some with MARD as well as MOD. Nevertheless, for the subgroup of patients presenting with robust insulin deficiency (SIDD) it is concluded that they might have advantageous actions from the present second-line therapy for T2D. As the SIDD group is correlated with a lesser BMI, no particular antidiabetic agents are considered superior over others for these patients that need rectification of weight.
Those patients belonging to the category of robust or Severe Insulin Resistance Diabetes (SIRD), that present with greater BMI in addition to the existence of greater BMI along with greater chances of coexistence of NAFLD, might have preference of treatments which result in weight reduction in addition to cause enhancement of insulin sensitivity. For these such groups of agents are SGLT2 inhibitors in view of their potentially resulting in escalated insulin sensitivity, as well as clinically significant reductions of body weight .It is still not clear if GLP-1RA treatment would prove to be advantageous in this group in view of restricted Clinical trials that have evaluated insulin sensitivity. Nevertheless, since GLP-1RA cause reduction of weight in addition to hepatic lipid amounts, they might be promising for SIRD. Further pioglitazone treatment is also efficacious for escalated insulin sensitivity in addition to NAFLD reduction. Nevertheless, it needs to be thought of only when no other treatment modalities are available, in view of the proven weight increments in addition to other side actions that are correlated with pioglitazone delivery. In this group apparently DPP-4 Inhibitors do not seem to have any therapeutic part in view of no proven actions on insulin sensitivity, decrease in weight, or NAFLD reduction.
The patients belong to the Mild Obesity-associated Diabetes (MOD) possessing the properties of moderate IR along with little deficiency of insulin, but a greater BMI. They might just need metformin monotherapy; however in case glucose amounts remain uncontrolled, for those SGLT2 inhibitors as well as GLP-1RA treatment might give benefits, since both groups result in significant weight reduction. In these groups it is preferable to prevent use of pioglitazone in view of the associated side effects of weight gain.
As far as patients belonging to the Mild Age-Related Diabetes (MARD) possess the properties of moderate IR in addition to little deficiency of insulin, greater age on diagnosis as well as lower chances of end organ injury, sulfonylurea along with DPP-4 Inhibitors might be the best modalities as add on therapies to metformin, in case metformin alone can’t control the hyperglycemia. Nevertheless, SGLT2 inhibitors as well as GLP-1RA treatment might also be the modality for MARD patients having proven end organ damage like Cardiovascular Disease (CVD) in addition to decreased kidney function. As the population here are older, the final decision have to be made by precision as well as considering adverse effects of every medicine needs to be taken into account.
For managing to perform successful T2D treatment, here we took into account that significant percentage of patients would need extra medicines, besides lifestyle modifications along with metformin therapy. The subgroups addressed by Ahlqvist in addition to the accepted metabolic actions on beta cell function along with insulin sensitivity of the separate classes of antidiabetic medicines might aid in giving a more individualized type of treatment for T2D patients depending on the major root causes of hyperglycemia in each person as addressed above [56]. Nevertheless, the ultimate therapy decision in every T2D person needs to account for other parameters that are correlated with Diabetes. Like in the existence of CVD, SGLT2 inhibitors or GLP-1RA treatment might be the accepted choice, without accounting for their correlated subgroups. Other parameters like existence of Diabetic Kidney Disease (DKD), the significance of weight reduction combined with lifestyle modifications, age of the patient, and patient’s choice in addition to probable adverse actions need to be accounted for.
We understand that Ahlqvist trying to cluster sub subgroups considering the metabolic phenotype of T2D might not turn out to be the ultimate Diabetes classification, with greater evaluations are required. Moreover at present no intervention trials that exhibit scientific proof that points that which particular antidiabetic medicines is the most efficacious for patients on the basis of their metabolic phenotype. Hence the advice detailed here are just the posit development as well as should not be considered to be recommendations. For proving the most proper treatment, subsequent trials are required in Diabetes subgroups to give a scientific basis for generation of individualized medicine for treatment of large as well as variable populations of T2D patients.
Citation: Kaur KK, Allahbadia G, Singh M (2021) Updating the Classification of Type 2 Diabetes Mellitus Subgroups by Ahlqvist for Achievement of Individualized Treatment Approaches for greater DM Control from Initiation and Avoidance of End Stage Damage. Pancreat Disord Ther. 11: 218.
Received: 05-Oct-2021 Accepted: 19-Oct-2021 Published: 26-Oct-2021
Copyright: © 2021 Kaur KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.