Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2014) Volume 3, Issue 2
Semen analysis of 1000 samples was evaluated to ascertain the male factor involvement in the infertility challenge. The Sperm Quality Analizer Visual (SQA-V) - a CASA protocol was used. This technology generates comprehensive semen analysis result in just 75 seconds. Of the 1000 samples analyzed, only 169 (16.9%) had all normal parameters; while the remaining 831 (83.1%) had abnormal parameters detected from them. Of 606 samples, 357 (58.9%) were Asthenozoospermia; 307 (50.7%) were Necrozoospermia; 101 (16.7%) were Oligoasthenozoospermia; 487 (52.0%) of 937 samples were Oligozoospermia; 172 (17.2%) of the 1000 samples were Hypospermia; 160 (16.0%) were Normozoospermia; while only one (0.2%) of 602 samples was Teratozoospermia. Seminal analysis is assessment of generational continuity. Couples experiencing infertility challenge in their marriages are encouraged to consult with their doctors’ early enough; avail themselves the opportunity for proper medical laboratory services while still relying on the Almighty God for divine intervention.
<Keywords: Semen; Analysis; Diagnostic value; SQA-V; Infertility; Nigeria
Seminal analysis evaluates certain characteristics of the male’s semen and spermatozoa contained therein. The most common reasons for medical laboratory semen analysis in humans are as part of a couple’s infertility investigation and after a vasectomy to verify that the procedure was successful.
The ‘World Health Organization 2013’, defines infertility as the inability of a couple to achieve pregnancy after twelve months of contraceptive-free intercourse. Infertility is often not seen (by the West) as being an issue outside industrialized countries [1,2]. This is because of assumptions about overpopulation problems and hyper fertility in developing countries, and a perceived need for them to decrease their populations and birth rates [3]. Despite this, infertility has profound effects on individuals in developing countries, as the production of children is often highly socially valued and is vital for societal security and health networks as well as for family income generation [3]. Infertility in these societies often leads to social stigmatization and abandonment of spouses [1]. The consequences of infertility are manifold and can include societal repercussions and personal suffering [4]. Infertility is, in fact, common in sub-Saharan Africa. Unlike in the West, secondary infertility is more common than primary infertility, being most often the result of untreated STIs or complications from pregnancy/birth [5].
Hudson stated that, about 40% of the issues involved with infertility are due to the man, another 40% due to the woman, and 20% result from complications with both partners [6]. However, data from UK in 2009 revealed otherwise; and that 30% of the infertility causes are attributed to the male, 30% to the female, 10% combined, 25% unexplained and 5% others [7]. Male infertility refers to the inability of a male to achieve pregnancy in a fertile female. In humans it accounts for 40- 50% of infertility [8-14]. Male infertility is common due to deficiencies in the semen, and semen quality is used as a surrogate measure of male fecundity [10]. Male infertility may be caused by pre-testicular causes [11,12], such as tobacco smoking [13-15], DNA damage [16], testicular factors [17,18], and post-testicular causes. Post-testicular factors decrease male fertility due to conditions that affect the male genital system after testicular sperm production and include defects of the genital tract as well as problems in ejaculation.
The traditional method of semen analysis (manual technique), a tedious and time consuming methodology with about ten parameters measures only some of the parameters in semen quality. The SQA-V protocol (automated system) commonly referred to as Computer- Assisted Semen Analysis (CASA) measures over twenty-one parameters. Computer-assisted techniques are the most-often used for the assessment of sperm concentration and mobility characteristics, such as velocity and linear velocity. Functional sperm concentration is also assessed. Computer-Assisted Semen Analysis (CASA) system is based on image analysis and using new techniques, with near perfect results, and doing full (comprehensive) analysis in a few seconds [19,20].
The aim of this work therefore is to reveal the gains associated with using Computer-Assisted Semen Analysis (CASA) protocol in a country were a very large number of Medical Laboratories still use the manual method in assessing semen quality. The objective on the other hand is to put on record the Medical Laboratory perspective of the current state of infertility associated with the male factor in the Federal Capital Territory of Nigeria.
Subjects
Semen samples from one thousand male partners from infertile couples were included in the study. A large number of these clients were actually sent by their physicians. All the samples were examined at the Medical Microbiology Laboratory of National Hospital, Abuja Nigeria.
Semen collection
For the analysis of these samples, clients were instructed to collect acceptable samples by masturbation method [29].
SQA-V procedure
Quality control: The system is self-testing and self-calibrating and runs latex beads or stabilized sperm quality controls. The system passes the self-test before samples are being analyzed. When the system is turned-on, it runs an internal self-check programme. If the system is okay, a pass report is displayed on the screen. Before replacing the manual technique with the SQA-V method of seminal analysis, several samples were analyzed with both methods; repeat test on same sample gave acceptable reproducible results. We equally run periodic manual methodology of some parameters on samples to ascertain and quality control the system. Good accuracy of the SQA-V method was established following repeated tests to the semen samples which gave accurate and reproducible results. The Papanicolaou staining method was used to assess the accuracy of the SQA-V machine for sperm morphology.
Semen analysis: The samples were analyzed in accordance with WHO guidelines for processing semen; though using the SQA-V methodology (Computer- Assisted Semen Analysis – CASA - protocol). The WHO guidelines for the performance of seminal analysis include the following;
1. Number of days of abstinence (2-7 days) before collection of sample
2. Method of collection of semen
3. Time of production to test (30-60 minutes)
4. Measurement of volume of sample
5. Liquefaction time (normally between 30 and 60 minutes)
6. Viscosity rating
7. Assessment of WBC concentration
8. Reference values
The SQA-V Capillary is filled with about 0.6 ml of completely liquefied semen sample by pushing the syringe piston in fully. All semen should be removed from the exterior of the capillary with cotton wool to prevent spillage into the SQA-V chamber. Manipulation of the SQA-V machine is according to manufacturer’s instruction.
Semen samples were examined between 30-60 minutes of their collection for volume, appearance, liquefaction, odour, pH, viscosity and WBC concentration.
WBC Concentration Evaluation Method
The concentration of WBC was determined in a wet preparation by multiplying the number of WBC with a known factor based on the size of a microscope field and the height between the objective glass slide and the cover slip (or the depth of the semen sample). The diameter of the microscope field can be measured using a micrometer.
The surface area in one field is equal to the square of the radius multiplied with pii (S=πr2).
E.g. Diameter = 250 μm → radius = 125 μm
Surface area = πr2 = 3.142 x 1252 = 3.142 x 15625 = 49093.75 μm2
The distance between the object glass slide and the cover glass (slip) can be calculated using the formula:
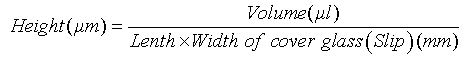
Volume of the sample = 20 μl
Length & Width of Cover glass (slip) = 22×22 mm
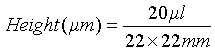
Knowing these figures a factor can be determined using this formula:
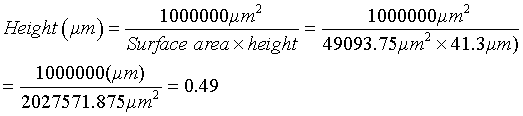
This means that if 3 WBC’s are counted in one microscope field, the corresponding concentration would be 1.47 million/ml (3×0.49). * We used the average of WBCs seen in ten microscope fields. The threshold value for a normal seminal WBC count is 1×106/ml [21].
Parameters Tested with SQA-V
Sperm concentration, Motility (PR+NP), Progressive Motility (PR), Non-Progressive Motility (NP), Immotility (IM), % normal morphology, Motile Sperm Concentration (MSC), Progressively Motile Sperm Concentration (PMSC), Functional Sperm Concentration (FSC), Average Velocity (VELOCITY), Total Sperm Concentration (TSC), Post-vasectomy test results - # motile sperm, # immotile sperm, # total sperm, # motile sperm/Vol, # immotile sperm/Vol, # total sperm/Vol.
For clients that also had request for semen culture, they were instructed appropriately on how best to collect samples to avoid bacterial contamination. The time between collection of the semen sample and the start of cultural proceedings should not exceed 3 hours; but should be after complete liquefaction. Semen samples were cultured appropriately while bacterial growth was examined using standard bacteriological techniques and bacterial identification was by appropriate identification methods.
The laboratory result of the 1000 samples is presented in Table 1. Of the 465 samples with normal sperm count, 160 (34.4%) had all the assessed parameters normal while 305 (65.6%) had one, two or even more abnormal parameters as shown in Table 2. The abnormal parameters detected were; low sperm motility rate, low progressive sperm motility rate, abnormal liquefaction, abnormal viscosity and low semen volume.
| Class of Infertility | Number Encountered | Normal Count | Low Count | Acute Pathological Sample | Azoospermia |
|---|---|---|---|---|---|
| (15×106/ml) (39×106/ ejaculate) | (5- <15×106) | (<5×106) | |||
| 1° Infertility | 258 | 107 (41.5%) | 37(14.3%) | 93 (36.1%) | 21 (8.1%) |
| 2° Infertility | 188 | 93 (49.5%) | 25 (13.3%) | 64 (34.0%) | 6 (3.2%) |
| 1° or 2° Infertility | 554 | 265 (47.8%) | 79 (14.3%) | 174 (31.4%) | 36 (6.5%) |
| Total | 1000 | 465 (46.5%) | 141(14.1%) | 331 (33.1%) | 63 (6.3%) |
Table 1: All Categories of Infertility.
| Nature of Abnormality / Terminology | Number Encountered |
|---|---|
| One abnormality(Monoabnormospermia) | 32 (10.5%) |
| Two abnormalities(Doubleabnormospermia) | 148 (48.5%) |
| Three abnormalities (Tripartiteabnormospermia) | 57 (18.7%) |
| Four abnormalities (Quartetabnormospermia) | 65 (21.3%) |
| Five abnormalities(Pentaabnormospermia) | 3 (1.0%) |
Table 2: Nature of Abnormalities from 305 samples.
Due to the high rate of semen samples with abnormalities, we also considered cases with moderate low sperm count. Of the 76 cases in this category, 33 (43.4%) had the adequate total number of spermatozoa per ejaculate; this was compensated by the increased volume of semen produced. Of this 33 with moderate low sperm count (10-14.9×106 spermatozoa per millilitre) cases but with adequate total (39×106) spermatozoa per ejaculate, 9 (27.3%) had all the parameters normal; 8 (24.2%) had one abnormality (Monoabnormospermia); 12 (36.3%) had two abnormalities (Doubleabnormospermia); 2 (6.1%) had three abnormalities (Tripartiteabnormospermia); while 2 (6.1%) had four abnormalities (Quartetabnormospermia) detected in their semen samples.
Culture results of the 392 semen samples revealed three categories; 5 (1.3%) had pathogens isolated; 194 (49.5%) had no pathogens Isolated; while 193 (49.2%) yielded no bacterial growth. The five pathogens isolated were Enterococus species, Escherichia coli, Klebsiella species, Staphylococcus aureus and Streptococcus species.
It is rather alarming to note in this study that, a high percentage of semen samples had either one, two, three, four or even five abnormalities. We however noticed that, all the semen samples analyzed had more than the required normal morphology of 4%; except for the acute pathological cases that had less than 5×106 spermatozoa per millilitre. A normal sperm count does not necessarily imply that all is well; hence detailed analysis was carried out for this group of clients. Of the 465 samples with normal sperm count, 160 (34.4%) had all the assessed parameters normal; while 305 (65.6%) had one, two, or even more abnormalities as shown in table 2. A single abnormal parameter (monoabnormospermia) identified or detected in a semen sample could be capable of being an obstacle to the spermatozoa from getting to their final destination (the ovum).
When the sperm motility rate or progressive sperm motility rate is compromised; a large number of the spermatozoa would have died along the way (the fallopian tube); even the remaining ones would have lost their viability and so unable to penetrate the ovum. Semen samples that fail to liquefy between 30-60 minutes (abnormal liquefaction) tend to hold the spermatozoa together, hence majority would die and the remaining live ones would be unable to travel to their final destination because they are locked-up together in what we may refer to as “spermatozoa detention”. Abnormal viscosity also operates in like manner – the slimy nature of the semen sample holds back the spermatozoa in a “string-back fashion”; thereby, failing to release the spermatozoa. A sizable number of the samples with abnormal viscosity were actually hyper viscous (hyper viscid); even after 90-120 minutes; and up to 240 minutes in some cases. The few cases of low semen volume actually affected the total sperm number per ejaculate.
In all, 169 (16.9%) cases had normal parameters encountered from the 1000 samples analyzed. It therefore mean that, the remaining 831 (83.1%) had one form of abnormality or more. This high rate of samples with low sperm quality and associated with the male factor is so alarming, and hence requires urgent attention. This very high rate could be as a result of the use of the CASA methodology which is more accurate than the manual technique.
In Nigeria, various researchers have reported varying degrees of abnormalities associated with the male factor. In 1981, Ajabor et al. reported a 57.5% semen abnormality as observed in Benin, South South Nigeria [22]. Adetoro & Ebomoyi (1991) reported 45% abnormality from Ibadan [23]. Idrisa et al. (2001) reported 70% as attributable to male factor in Maiduguri [24]. In 2003, Ikechebulu et al. had reported 42.4% abnormality as being that of the male factor in Nnewi and Awka in the South Eastern region of Nigeria [25]. In 2008, 70% was also reported from Abakaliki by Ugwaja et al. [26]. In 2013, Agu et al. reported 62.2% of semen samples analyzed in Kano having abnormalities in semen concentration [27]. However, Owolabi et al. also in 2013 reported a lower abnormal semen quality in 31.8% of male partners of couples seeking remedy for their inability to conceive in Ile- Ife, South West Nigeria [27]. On April 16th 2013, the Daily Post (Nigeria online Newspaper) reported that; fertility experts have warned that if nothing is done urgently to reverse the trend, more Nigerian men will not be able to impregnate their spouses as they blamed most cases of childlessness in marriages to male factor.
s
Semen culture results revealed that of the 392 samples cultured, only 5 (1.3%) had pathogens isolated from them such as Enterococus species, Escherichia coli, Klebsiella species, Staphylococcus aureus and Streptococcus species. Bacterial infection could therefore not be associated with the various cases of semen abnormalities resulting to the male factor. Our cultural findings are in great variance from that reported by, which had 74.9% of the samples having pathogenic organisms [27]. They incriminated Staphylococcus aureus as the most common organism isolated from the samples. Most of these Staphylococcus aureus isolates could have been skin contaminants as a result of poor or wrong specimen collection. Owolabi et al. (2013) also reported a high level of leucocytospermia in their study as against the 7.4% leucocytospermia reported by ‘Agu et al. 2013’ [27]. In this study, leucocytospermia was as low as 4.5%.
Our study also revealed that, from the 606 samples (465-Normal count; 141-low count) that had total sperm number per ejaculate, 357 (58.9%) were Asthenozoospermia; 307 (50.7%) were Necrozoospermia; 101 (16.7%) were Oligoasthenozoospermia. 487 (52%) of 937 samples were Oligozoospermia. 63 (6.3%) of 1000 samples were Azoospermia; 172 (17.2%) of the 1000 samples were Hypospermia; 160 (16.0%) were Normozoospermia; while only one (0.2%) of 602 samples was Teratozoospermia. Of the 329 samples with total sperm number per ejaculate but had one, two, three, four or five abnormalities, 40 (12.2%) were Monoabnormospermia; 160 (48.6%) were Doubleabnormospermia; 59 (17.9%) were Tripartiteabnormospermia; 67 (20.4%) were Quartetabnormospermia; while 3 (0.9%) were Pentaabnormospermia. Of course, all these cases have the capacity to cause infertility. Moreover, in cases where there is ‘Cervical hostility’ from the female partners, the conditions may be worse.
The use of CASA methodology with Sperm Quality Analyzer Visual (SQA-V) Machine (Figure 1) has so many advantages; Automatic semen analysis results in 75 seconds and has the ability to print test results and archive up to 500 patient results. The system is self-testing and selfcalibrating and runs latex beads or stabilized sperm quality controls. Two systems: automated and visualization allow the user the flexibility to analyze all types of semen samples. WHO semen parameters are reported in addition to derived and total/ejaculate parameters - all these SQA-V reported parameters result in a comprehensive semen analysis assessment; Automatically reads fresh, frozen, washed and post-vasectomy samples; On-screen visualization of the semen sample on the video screen of the SQA-V or on a PC display (with counting grid and image freezing) using a standard laboratory slide or an SQA-V capillary; Variable optical magnification from x300 to x500; Video clips can be recorded using V-Sperm III software; A complete semen analysis report can be automatically printed-out; A “High Sensitivity” test mode for oligo-, asteno- and azoospermia determination, and vasectomy validation; Disposable testing capillary is biologically safe and can be conveniently used in virtually any testing environment; PC-compatible; Patient test results, images and clips can be downloaded to a PC using V-Sperm III.
Owing to the increasing rate of abnormalities encountered in semen samples, we encourage couples having this infertility challenge in their marriages to avail themselves the opportunity for proper medical laboratory services to seek for solution while still relying on the almighty God for the fruit of the womb. This aspect of seeking divine intervention becomes imperative because some men are born that way – as Eunuchs (Holy Bible, Matthew 19:12). However, our God is a prayer answering God; and of course there is nothing too hard for Him to do. So, there is hope in divine intervention.
As professionals (biomedical scientists, medical laboratory scientists); having this enormous responsibility to identify the true situation of clients in this category thereby enabling the physician or Andrologist for proper and effective patient management cannot but update and upgrade ourselves and by making use of available and modern technologies in the performance of our duties in this regard.
Dr. J. O. Ajobiewe (Ph.D), Medical Microbiology Laboratory, National Hospital, Abuja
Mr. Talle Mohammed, WHO National Polio Laboratory, UMTH, Borno State
Mrs. Osy Donbraye, Microbiology Laboratory, UCH, Ibadan
Mr. Ghandi Ogbopuru, Federal Medical Centre, Yenagoa, Bayelsa State
PELETIRI, I. C. – Research design, analysis, Research methodology, interpretation of data, performed the research, drafted the paper, critical revision, approval of the submitted and final version.
ALE, S.T. – Research methodology, performed the research, critical revision, approval of the submitted and final version.
PELETIRI, D. C. – Interpretation of data, drafted the paper (literature review), critical revision, approval of the submitted and final version.
The approval of the Ethics Committee in our institution was not necessary because samples used for this work were obtained from our clients sent with request forms for semen analysis from their physicians. As analyzers of the samples, we used the opportunity to share our experience with the global health community. The principles in the WMA Declaration of Helsinki – Ethical Principles for Medical Research involving human subjects were adhered to; none of the items directly relate to our research protocol. No potential conflicting interest. No research funding was received from any organization or institution. No informed consent was obtained because it was unnecessary.