
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2022)Volume 12, Issue 1
Uptake of Fluorescence-tagged Acid-functionalized Single-walled Carbon Nanotubes (FAF-SWCNTs) was examined in Peripheral Blood Derived Mononuclear Cells (PBMC) preparations derived from six patients of Acute B-lymphocytic Leukemia (ALL), five patients of Chronic B-lymphocytic Leukemia (CLL), and four healthy control blood donors. Mean percentage of B cells in PBMC samples was 14.08% (healthy controls), 56.50% (ALL), and 88.74% (CLL). PBMCs were incubated with FAF-SWCNTs for 24 h and uptake in CD19+ B cells was examined by flow cytometry. In all cases, significant uptake of FAF-SWCNTs was found in all CD19+ B cells. A subpopulation of B cells (6% in ALL samples, 3% in CLL samples) showed significantly greater uptake of FAF-SWCNTs. Cellular distribution of the FAF-SWCNTs internalized by B cells in different samples was also examined by confocal microscopy. All B cells internalized the FAF-SWCNTs that were essentially localized in the cytoplasmic region. Incubation of PBMCs with AF-SWCNTs resulted in a significant induction of Phosphatidyl Serine (PS) in ALL and CLL samples but not in PBMCs from healthy donors. Cell cycling studies by using PI staining followed by flow cytometric analysis indicated that the treatment with AF-SWCNTs caused a marked decrease in the S phase of cell cycle in ALL and CLL samples. These results suggest that the treatment with AF-SWCNTs induce cytotoxic and cytostatic effect on human B cell leukemia cells.
Carbon nanotubes; Human B cell leukemia; Apoptosis; Cell cycle; Chronic B-lymphocytic Leukemia; Flow cytometry; Confocal microscopy
Leukemia, a hematologic malignancy that originates from bone marrow, leads to lifelong morbidity and high mortality in patients [1]. Acute Lymphoblastic Leukemia (ALL) and Chronic Lymphoblastic Leukemia (CLL) are the most common types of leukemia [2]. Every year, millions of people are diagnosed with leukemia, and hundreds of thousands die due to a lack of adequate, effective treatment [3]. Leukemia alone contributes 3.5% of total new cancer cases in USA [3]. Though the etiology of leukemia is still unknown, the treatment has evolved with time. Treatment options include chemotherapy, monoclonal antibodies [4-6], Hematopoietic Stem Cell Transplantation (HSCT), and different types of inhibitors and chemotherapeutic agents [6-8]. None of the conventional therapies is however effective enough to completely cure leukemia.
Carbon Nanotubes (CNTs), have emerged as a new material with the potential of extended utilization in electronics and biomedical fields [9,10]. CNTs unique physical structure of spare electrons, nano dimension, and high aspect ratio allow it to be used in diagnostics, tissue engineering, drug delivery, gene therapy [11-15], and vaccine delivery, purpose [16-18]. As such Single-Walled CNTs (SWCNTs) are insoluble and hydrophobic and do not interact well with the biological systems [19]. Acid functionalized form of SWCNTs (AF-SWCNTs) are however hydrophilic and capable of interacting with immune cells [19-21]. Our group has been studying the interactions of AF-SWCNTs with the immune system both in vivo and in vitro. AF-SWCNTs modulate Hematopoietic Stem And Progenitor Cells (HSPCs) self-renewal and HSPCs to leukocytes ontogenesis [22], bone marrow and spleen erythropoiesis [23,24], NK/T cell activation [25], presentation of lipid and protein antigens [26,27], and alter epithelial tight junctions [28]. Our results also suggest that the activated T and B cells internalize a significantly higher amount of AF-SWCNTs; and are susceptible to their cytotoxic effects [29,30].
Many types of nanoparticles have been used for therapeutic purposes to treat leukemia [31-34]. Though carbon nanotubes interact with immune cells most efficiently [25,27-29,35], their use as possible therapeutic agents for leukemia remain unexplored. In the present study we have examined the uptake of AF-SWCNTs in vitro by B cells derived from control and B cell leukemia patients. Our results indicate that AF-SWCNTs are taken up by all activated leukemic B cells. Treatment with AF-SWCNTs induces apoptosis as well as a decline in the S phase of cell cycle in leukemia B cells. These results indicate that AF-SWCNTs may be a good candidate for leukemia therapy and their possible use may be further explored.
Collection and processing of human blood samples
The participants of the investigation were enrolled in Dr. B.R.A Institute-Rotary Cancer Hospital at the All India Institute of Medical Sciences, New Delhi. The investigation was approved by the competent authority at AIIMS (Ref. No. IEC-821/08.11.2019, RP-13/2019) and experiments were performed in strict compliance of institutional and ICMR guidelines. All participants provided informed written consent, to be a part of this study. In this preliminary study, four samples from healthy control donors (STN, M25; MR M27, EB M29, KH M27), six samples from ALL patients (DH F22, EY M25, SA M16, RE M12, TA F33, BH M44) and five from CLL patients (GA M59, SH M57, KS M58, AP F56, SS M65) were studied. Peripheral Blood (PB) samples were collected from healthy donors or B-ALL/B-CLL patients in K2-EDTA by on duty nurse and immediately transported to South Asian University for further study. In order to recover the Peripheral Blood Mononuclear Cells (PBMCs), Participant’s Blood Samples (3 ml) were diluted with equal volume of PBS containing 2% FBS. Lymphoprep (3 ml at 15-25°C) was aliquoted into 15 ml sterile plastic centrifuge tubes. Diluted blood cells suspension was carefully layered on top of Lymphoprep layer and the tubes centrifuged at 800 g for 20 minutes at room temperature (15-25°C). Human PBMCs in the buffy interphase were collected and washed twice with medium before use.
Reagents and other supplies
SWCNTs, 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide (EDAC), N-Hydroxysuccinimide (NHS), 2-(N-Morpholino) Ethane-Sulfonic Acid (MES) were purchased from Sigma Aldrich (St. Louis, MO). Centrifugal filter device centricon (3 kDa) and dialysis tubing (100 kDa) were procured from Millipore (Billerica, MA). Alexafluor 633 hydrazide was ordered from Molecular Probes (Carlsbad, CA). RPMI-1640 medium (with 2 mM glutamine, 1 mM sodium pyruvate, 4.5 g glucose/L, 10 mM HEPES, 1.5 g/L sodium bicarbonate and 20 ug/mL gentamycin) and Fetal Bovine Serum (FBS) were purchased from Gibco (Carlsbad, CA). Fluorochrome tagged anti-human CD19, Annexin V were purchased from Biolegend (San Diego, CA). DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride), Propidium Iodide (PI), Ribonuclease A (RNase A) were ordered from Invitrogen (Carlsbad, CA). Lymphoprep was from Axis-Shield PoC AS (Oslo, Norway).
Acid functionalization of SWCNTs
Acid-functionalization of SWCNTs was performed as described before [23]. Briefly, SWCNTs (Sigma, Cat#775535, >95% carbon) (20 mg) were suspended in concentrated H2SO4 and HNO3 mixture (1:1 ratio). They were subject to high pressure heat treatment in a microwave oven (20 ± 2 psi, 50% of 900 W power, temperature 135-150°C) for 3 minutes. Suspension was diluted (2X) in water, dialyzed against deionized water and lyophilized for further use. Zeta sizer study has indicated the AF-SWCNTs an average particle size (277.06 ± 3.37 d.nm) and an average zeta potential of -47.02 ± 0.69 mV (Supplementary Figures 1A and 1B). Nanotubes BET surface area was measured using flow BET nitrogen adsorption technique. SWCNTs and AF-SWCNTs BET surface area were 799.39 M2/g and 240.74 M2/g. Nanotubes were also examined under a Transmission Electron Microscopy (TEM). TEM study indicated that acid functionalization of carbon nanotubes significantly reduced agglomeration without disturbing the basic structure of the carbon nanotubes (Supplementary Figures 1C and 1D). Alexa fluor 633 were covalently tagged with AF-SWCNTs to obtain Fluorescent AF-SWCNTs (FAF-SWCNTs) as described before [26].
Flow cytometric assay
Cells to be analyzed were incubated with fluorochrome tagged anti-human CD19 antibody for 30 minutes in ice and washed with PBS+2% FBS. To study apoptosis, cells were suspended in 50 μl of Annexin Binding Buffer (ABB) containing fluorochrome tagged Annexin V for 30 minutes. For cell cycle study, cells were incubated with fluorochrome tagged anti-human CD19 antibodies and then fixed overnight at 4°C in 70% ethanol. Ethanol was removed by washing (3X) and the cells treated with Ribonuclease A (50 μg/mL, 2 h at 37°C) to denature RNA. Cells were washed once and treated with Propidium Iodide (PI) (5 μg/ml) in ice for 30 minutes. Flow cytometry samples were analyzed on FACSVerse or FACSAria III. Data were analyzed using FACSuit, FACSDiva, Flowjo and Modfit 5.0 softwares.
FAF-SWCNTs uptake by B lymphocytes
Protocol for the analysis of FAF-SWCNT uptake by confocal microscopy has been described elsewhere [19]. In brief human leukocytes were incubated with FAF-SWCNTs (20 μg/million leukocytes) in 1 ml culture medium in 48 well culture plates. Cells were harvested after 24 hours and stained with fluorochrome tagged anti-human CD19 antibodies. CD19+FAF-SWCNT+ cells were sorted by using FACSAria III cell sorter. Sorted cells were counter-stained with DAPI (100 ng/ml) and adhered onto poly-llysin (0.01%) coated coverslips. Coverslips were mounted on glass slides using prolong gold anti-fade reagent. Cells were analyzed at 100X magnification using A1R confocal laser scanning microscope (Nikon, japan).
Statistical analysis
Results are represented as Mean ± SEM. Statistical analysis was performed using Sigma plot software (Systat software, San Jose, CA). Student’s t test or ANOVA was used to calculate differences among groups. P-value <0.05 was accounted as significant (P<0.05*, <0.01**, <0.005***, <0.001****).
Determination of CD19 positive B cells percentage
To determine the proportion of B cells in peripheral blood, blood samples from healthy controls and leukemia patients were collected and PBMCs isolated as mentioned in the materials and methods section. Cells were stained with anti-human CD19 antibody and analyzed using flow cytometry.
Results in Figure 1 show the Mean ± SEM values for the proportions of B cells in control, ALL and CLL groups of samples. Mean CD19 positive B cells percentages in PBMC samples from healthy controls, B-ALL, B-CLL patients were 14.08%, 56.50%, and 88.74% respectively. As expected, proportion of B cells in peripheral blood of ALL and CLL patients was markedly raised as compared to healthy controls (P<0.001).
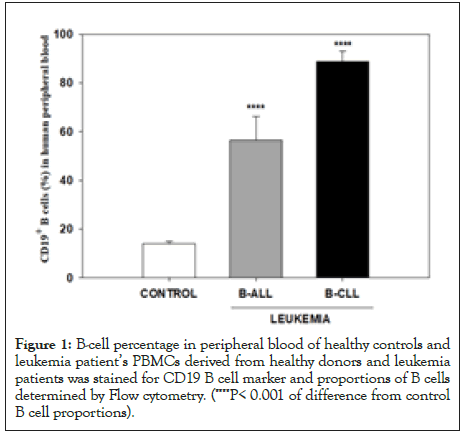
Figure 1:B-cell percentage in peripheral blood of healthy controls and leukemia patient’s PBMCs derived from healthy donors and leukemia patients was stained for CD19 B cell marker and proportions of B cells determined by Flow cytometry. (****P< 0.001 of difference from control B cell proportions).
FAF-SWCNTs uptake by B cells from healthy donors and B-cell leukemia patients
To assess FAF-SWCNTs uptake by B cells, healthy control, and leukemia patients PBMCs were incubated with FAF-SWCNTs followed by staining with anti-human CD19 antibodies. Representative histograms shown staining with FAF-SWCNT and CD19 antibody in Figure 2A. Upper right quadrangles (quadrangle B) showing B cells that had internalized FAF-SWCNTs, were further subdivided in to quadrangles P1 (high uptake) and P2 (low uptake). Figure 2B shows the proportions and Mean Fluorescence Intensities (MFI a measure of average relative uptake of FAF-SWCNT on per cell basis) for all groups of cell populations in quadrangles A to D as well as quadrangles P1, P2. A closer examination of data of FAF-SWCNT-uptake (quadrangles B, P1 and P2) show that in ALL patients had a discrete population of B cells (6.2% in quadrangle P1) that internalized significantly higher amounts of FAF-SWCNTs as compared to healthy control samples (0.8% in quadrangle P1). In CLL samples the increase was less (3.5% in quadrangle P1). MFI values for quadrangles P1 and P2 indicate that the mean relative per cells uptake of FAF-SWCNTs was 15191, 32316 and 23525 respectively. Our results thus show that while almost all B cells in PBMCs of healthy controls as well as ALL and CLL groups internalized significant amount of FAF-SWCNTs, a subpopulation of B cells in ALL and CLL patients internalized significantly higher amounts of FAF-SWCNTs as compared to control cells.
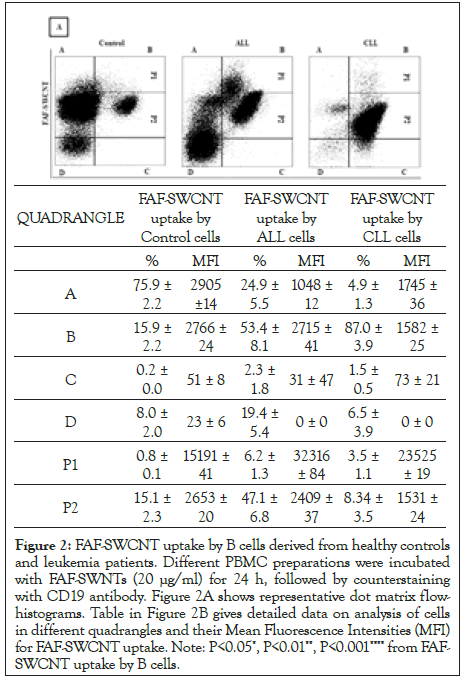
Figure 2: FAF-SWCNT uptake by B cells derived from healthy controls and leukemia patients. Different PBMC preparations were incubated with FAF-SWNTs (20 μg/ml) for 24 h, followed by counterstaining with CD19 antibody. Figure 2A shows representative dot matrix flowhistograms. Table in Figure 2B gives detailed data on analysis of cells in different quadrangles and their Mean Fluorescence Intensities (MFI) for FAF-SWCNT uptake. Note: P<0.05*, P<0.01**, P<0.001**** from FAFSWCNT uptake by B cells.
FAF-SWCNTs localization in B lymphocytes
For visualization of the intracellular distribution of FAF-SWCNTs, control, ALL and CLL PBMCs were incubated with FAF-SWCNTs (20 μg/ml for 24 hours), counterstained with CD19 and CD19+FAFSWCNT+ cell population isolated by cell-sorting on FACS Aria III cell sorter. Purified CD19+FAF-SWCNT+ sorted cell populations were examined by confocal microscopy. The fluorescent intensity of FAF-SWCNTs was highest in B-ALL, followed by B-CLL and healthy control, which was in concurrence with our flow cytometry data. Z-section analysis shows that in all samples, the internalized FAF-SWCNTs (red fluorescence) was essentially localized in the cellular cytoplasm as the nuclear areas remained relatively dark (Figure 3).
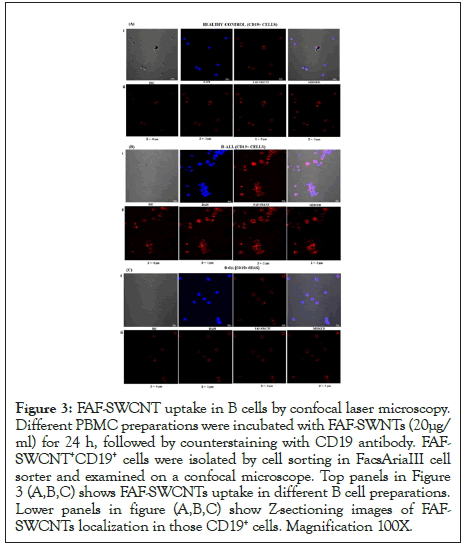
Figure 3: FAF-SWCNT uptake in B cells by confocal laser microscopy. Different PBMC preparations were incubated with FAF-SWNTs (20μg/ ml) for 24 h, followed by counterstaining with CD19 antibody. FAFSWCNT+ CD19+ cells were isolated by cell sorting in FacsAriaIII cell sorter and examined on a confocal microscope. Top panels in Figure 3 (A,B,C) shows FAF-SWCNTs uptake in different B cell preparations. Lower panels in figure (A,B,C) show Z-sectioning images of FAFSWCNTs localization in those CD19+ cells. Magnification 100X.
Cytotoxicity and cytostatic effects of AF-SWCNTs
Externalization of Phosphatidyl Serine (PS) in the outer cellular membranes is an indication of an apoptotic response [36]. Results in Figure 4 show that treatment of PBMCs from ALL and CLL patients with AF-SWCNTs resulted in a significant increase in the proportion of B cells that expressed PS. These results indicate that AF-SWCNTs induced a significant apoptotic response in a subpopulation of B cells from ALL and CLL patients.
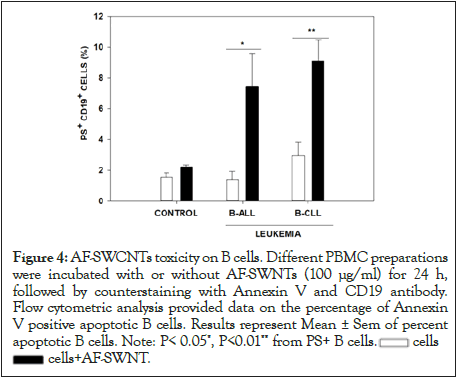
Figure 4:AF-SWCNTs toxicity on B cells. Different PBMC preparations
were incubated with or without AF-SWNTs (100 μg/ml) for 24 h,
followed by counterstaining with Annexin V and CD19 antibody.
Flow cytometric analysis provided data on the percentage of Annexin
V positive apoptotic B cells. Results represent Mean ± Sem of percent
apoptotic B cells. Note: P< 0.05*, P<0.01** from PS+ B cells. cells
cells cells+AF-SWNT.
cells+AF-SWNT.
Cell cycling analysis of B cells in control as well as ALL and CLL samples was also carried out. Results in Figure 5 show that while only 1.7% B cells were in S phase of cell cycle, the corresponding figures for ALL and CLL samples were 12.7% and 18.7% respectively for ALL and CLL samples. An increase in S phases in an indicator of increased cell proliferative activity in B cells from ALL and CLL samples. Further, in all cases, a fall in proportion of B cells in S-phase of cell cycle fell upon treatment with AF-SWCNTs. The decline was 1.7% to 0% in control cells, 12.7% to 4.75% for ALL samples (P<0.05) and 18.7% to 5.5% for CLL samples. The decline was however not statistically significant in CLL samples though this may be due to less number of samples that we studied. These results suggest that besides a cytotoxic effect, AF-SWCNTs may also have a cytostatic effect on B leukemia cells.
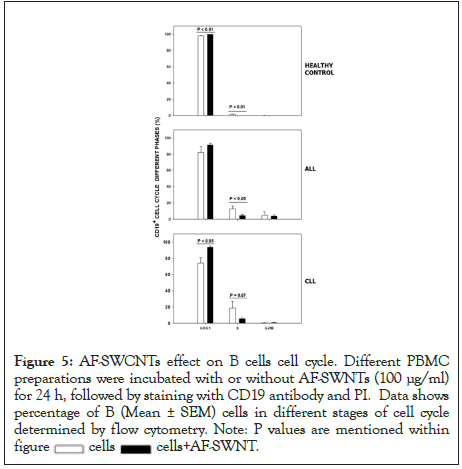
Figure 5:AF-SWCNTs effect on B cells cell cycle. Different PBMC
preparations were incubated with or without AF-SWNTs (100 μg/ml)
for 24 h, followed by staining with CD19 antibody and PI. Data shows
percentage of B (Mean ± SEM) cells in different stages of cell cycle
determined by flow cytometry. Note: P values are mentioned within figure  cells
cells  cells+AF-SWNT.
cells+AF-SWNT.
Our earlier studies have shown that Acid-Functionalized Poly- Dispersed Single-Walled Carbon Nanotubes (AF-SWCNTs) interact efficiently with immune cells and tend to suppress their functions [23,25,27-30]. We also noted that in general the uptake of AF-SWCNTs was significantly higher in activated B and T cells as compared to resting control B and T cells [29,30]. We also demonstrated in B cells activated in vivo by administration of LPS in mice, internalized significantly higher amounts of AF-SWCNTs and were susceptible to the cytotoxic effect of AF-SWCNTs. These results suggested that AF-SWCNTs could be a potential therapeutic agent to ablate activated B and T cells. This proposition was further tested in Graft-Versus-Host Disease (GVHD) mouse model. GVHD results from activation of activated immune cells of parental MHC haplotype when administered in F-1 mice [37]. We demonstrated that the administration of AF-SWCNTs in mice undergoing GVH reaction resulted in a marked downregulation of the GVH reaction, indicating that AF-SWCNTs could deplete activated T cells in vivo [28]. On the basis of these results, we posed the question whether AF-SWCNTs could also kill leukemia cells since these cells too represent a form of activated and proliferating. As a first step in testing this proposition, we have in the present preliminary study, examined the uptake of AF-SWCNTs and its possible cytotoxic effects on B cells derived from ALL and CLL patients. It must be mentioned here that we have essentially focused on B cells in PBMCs of B-cell leukemia patients and healthy controls and have not per se analyzed only leukemia cells. This is because there is no easy way in discriminating between healthy B cells and leukemic B cells in the PBMCs of leukemia patients, and further that the CD19 positive B cells from ALL and CLL patients are predominantly leukemia cells.
In B cells derived from ALL and CLL patients, uptake of AFSWCNTs was significantly higher in a minor subpopulation of B cells in these samples. This subpopulation of B cells that internalized more AF-SWCNTs was more prominent in ALL samples. Why only a small subpopulation of leukemic cells had higher uptake of AF-SWCNTs is not clear. It is possible that the increased uptake of AF-SWCNTs happens during a certain phase of cell cycle only, though this proposition needs to be further examined.
The biological consequences of internalization of AF-SWCNTs in control and leukemic B cells, be it low or relatively higher uptake, are not known. Our results show that AF-SWCNTs induced significant cytotoxic as well as cytostatic effects in leukemic B cells and this effect was substantially higher in leukemic B cells as compared to B cells from healthy donors. Our results thus demonstrate that the B cells from ALL and CLL samples may be more susceptible to the cytotoxic and cytostatic effects of AF-SWCNTs. Further work will be needed to determine if AF-SWCNTs could be considered a potential therapeutic agent in B cell leukemia patients.
Authors report no conflict of interest.
This research work was supported by Department of Science and Technology, Government of India, Nano-sciences Mission grant number SR/NM/NS-1219 and JC Bose award to RKS. MBM received fellowship support from the South Asian University.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Mia B, Pushpam D, Pushpam S, Saxena RK (2022) Uptake of Poly-dispersed Carbon Nanotubes Induce Cytotoxic and Cytostatic Effect on Human B Cell Leukemia Cells. J Clin Toxicol. 12:504.
Received: 24-Jan-2022, Manuscript No. JCT-22-15970; Editor assigned: 26-Jan-2022, Pre QC No. JCT-22-15970 (PQ); Reviewed: 07-Feb-2022, QC No. JCT-22-15970; Revised: 14-Feb-2022, Manuscript No. JCT-22-15970 (R); Published: 21-Feb-2022 , DOI: 10.35248/2161-0495-22.12.504
Copyright: © 2022 Mia B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.