
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2023)Volume 13, Issue 4
Rationale: Bronchopulmonary Dysplasia (BPD) is the major pulmonary morbidity in preterm infants, with the defining criteria for this condition changing several times. Perinatal Ureaplasma infection has been implicated in BPD risks and known to be prevalent in Neonatal Intensive Care Unit (NICU) patient populations. Some studies have suggested the use of Azithromycin can reduce BPD risks in Ureaplasma positive cases.
Objectives: The goal of this study was to conduct a secondary data analysis using a completed clinical trial to compare the incidence and severity of BPD in preterm infants using multiple BPD definitions.
Methods and results: Secondary data analysis including 220 preterm infants enrolled at birth and randomized to receive Azithromycin or placebo daily up to 6 weeks was evaluated. Categorical variables were compared using Pearson Chi-square or Fisher’s Exact tests, as appropriate. Distributions of BPD grade outcomes and the benefits of Azithromycin using four historically different scoring systems (VON-1988, NIH-2001, NICHD-2018, and Jensen-2019) on the same patient data set were compared. Among 176 survivors of this population, grade II/moderate BPD was significantly different at 43.8% and 47.1% compared with 17% according to NIH- 2001, Jensen-2019 and NIH-2018 definitions, respectively. In addition, shifts in BPD grades according to these classifications were seen in grade III/severe BPD, significantly different at 35.8%, 35.8% and 9% according to NIH-2001, NIH-2018 and Jensen-2019 definitions. Only VON-1988 and NIH-2001 BPD grading resulted in an association of Ureaplasma positivity with BPD severity and Azithromycin treatment, showing overall reduction in BPD outcomes. In contrast, the two most recent BPD scoring systems showed no statistically significant differences between Ureaplasma positive vs. negative cases nor azithromycin benefits.
Conclusion: The incidence of BPD, a major morbidity of premature birth, differs based on the definition applied. The impact of Ureaplasma positivity on BPD outcomes, and the potential benefit of Azithromycin in patients with this infection, were also highly dependent on the BPD grading system employed. This issue has a great effect on clinical trial design to address consistency of diagnosis in BPD. The study illustrates how crucial BPD grading is for defining potential causative agents and therapeutic strategies.
Bronchopulmonary dysplasia; Preterm infants; Ureaplasma; Azithromycin; Pulmonary morbidity
According to Centers for Disease Control and Prevention (CDC), approximately 10% of infants are born premature in the United States of America annually. Survival of preterm infants has improved in recent years, especially in cases of extreme prematurity within well-developed healthcare settings; however, the incidence of most major morbidities related to prematurity have slowly improved [1,2]. The majority of drugs used in Neonatal Intensive Care Units (NICU’s) are used off-label, and it is important for development of drug information and therapeutic strategies unique to conditions in neonates {FDA Clinical Pharmacology Guidance document, July 2022, Docket#: FDA-2019-D-3132}. Drug development and clinical trials involving preterm infants lag behind all other paediatric age groups in resource allocation. With the increased number of surviving preterm infants, there is a clear and prevalent need for rationally designed trials to identify safe and effective treatment [3].
One of the most common complications of premature birth is Bronchopulmonary Dysplasia (BPD), a serious lung disease related to immature lung structure and function, hyperoxic exposure, and traumatic lung injury associated with mechanical ventilatory support. BPD is a major cause of morbidity and mortality of preterm infants. There are high risks of lifetime disability, along with social and medical costs for survivors. Incidence of BPD ranges from 6%-57% in preterm infants, based on the clinical definitions used and extent of prematurity at birth [4]. Despite advancement in medicine and healthcare over the past few decades, BPD diagnoses have increased, with higher rates of survival among extremely preterm infants at Gestational Ages (GA) of less than 28 weeks. More than 50% of extremely preterm infants are affected by at least moderate BPD [2], and the current lack of preventive and effective treatment for BPD increases the burden of neonatal morbidity, mortality, and neonatal care cost.
A strong barrier in the development of an effective therapy for BPD is the lack of a universal consensus definition and severity classification of this lung disease. The definition of BPD and participating infant population, owing to better survival at lower gestational ages, has evolved since first described in 1967 [5]. In 1979, BPD was defined based on lung radiologic changes and the need for oxygen supplementation at 30 weeks of GA [6]. In 1988, a definition of BPD was proposed which diagnoses BPD with the requirement for oxygen supplementation at 36 weeks PMA for infants born at <1500 g [7]. In 2001, a newer strategy derived from the recommendation at an NIH workshop in 2000 divided BPD severity into 3 classes: mild, moderate, and severe [8]. In 2018 and 2019, two additional strategies have also been proposed and adapted by NICUs worldwide.
The etiology of BPD is not clearly defined, but current evidence supports multiple contributing factors. Underdeveloped lung structure related to premature birth is the cardinal factor; others include surfactant deficiency, pulmonary barotrauma, hyperoxia, and inflammation. Growing evidence has also linked bacterial infection, specifically Ureaplasma, with pulmonary disease in newborns [9,10]. Ureaplasma is a commensal organism in the genital tract of women. This pathogen is the most commonly isolated organism from infected placentas and consistently implicated in several obstetric complications including infertility, prematurity, neonatal morbidity, and perinatal mortality [11-13]. Multiple studies have shown the respiratory tract of preterm infants with BPD containing colonized Ureaplasma urealyticum, known to be prevalent in NICU patient populations [14-18]. Some studies have suggested Azithromycin, a macrolide antibiotic, can prevent or reduce BPD risks in Ureaplasma positive cases [19,20]. Additionally, there is evidence the use of Azithromycin in preterm infants may decrease the incidence of BPD [21]. The need for a primary outcome variable with focus on Ureaplasma positive cases and BPD in a full-scale randomized, placebo-controlled, clinical trial is novel and pressing.
The goals of this study were to utilize a well-defined data resource to compare pulmonary outcomes and define the relationships of Ureaplasma spp. colonization. In addition, using each of the four most common working definitions of BPD grade (VON-1988, NIH-2001, NICHD-2018, and Jensen-2019), measurement of Azithromycin use may benefit BPD outcomes in preterm infants. By conducting this secondary data analysis of a completed clinical trial at our institution, our intent was to maximize our insight for design and development of further trials to address this major morbidity in preterm infants.
Study design and human subjects
Data derived from a previously reported clinical trial [20], from 220 preterm infants enrolled at the University of Kentucky NICU, were used in a secondary analysis. Patients were previously randomized, double-blinded, placebo-controlled, and approved by the Institutional Review Board at the University of Kentucky. Eligibility criteria included birth weight equal or less than less than 1,250 g, mechanical ventilation equal or less than 12 hr duration, and age equal or less than 72 hrs. Each qualifying infant was enrolled at birth and randomized to receive Azithromycin or placebo for a total of 6 weeks. Table 1 summarizes general characteristics of the patients from this trial (more detail provided in reference) [20].
| Patients Characteristics (n=220) | ||
|---|---|---|
| Body weight, g | 807 ± 180 | |
| Gestational age, week | 25.9 ± 1.6 | |
| Male, no. | 108 | |
| Female, no. | 112 | |
| Race, no. | Black | 21 |
| White | 191 | |
| Other | 8 | |
| Cesarean Section, no. | 144 | |
Table 1: Provides an overview of the clinical characteristics of the trial participants.
Ureaplasma positivity was confirmed by PCR testing to detect the presence of Ureaplasma genes in tracheal aspirates, collected at enrollment on day 3 of treatment and weekly thereafter while intubated and on study. Major morbidities or death, plus pulmonary outcomes (BPD grade) were determined for each patient.
With this extensive data set, each patient was re-assessed with respect to BPD outcome using four varying definitions and grading systems that have been published and widely used in the literature. Each of these grading systems are described below:
VON-1988: The Vermont Oxford Network definition of BPD includes 2 categories, along with the requirement of oxygen supplementation at 36 weeks of PMA for infants born at<1500 g [7]. This system is still widely used by the VON consortium for institutional benchmarking of neonatal intensive care units.
NIH-2001: The NIH-2001 definition of BPD includes 3 categories of mild, moderate or severe. Infants defined to have BPD if gestational age is <32 weeks and required O2 support for more than 28 days, or corrected gestational age of 36 weeks but if>32 weeks required O2 support for 56 day old, or discharge home. At this time, if the infant is in room air, it is mild BPD. FiO2 <30% defines moderate BPD and FiO2>30% defines severe BPD.
NICHD-2018: The NICHD-2018 definition of BPD includes 3 categories for premature infants <32 weeks’ gestational age of grades I, II, or III. This definition includes persistent parenchymal lung disease, radiographic confirmation of parenchymal lung disease, and at 36 weeks PMA requires 1 of the following FiO2 ranges/oxygen levels/O2 concentrations for ≥ 3 consecutive days to maintain arterial oxygen saturation in the 90%–95% range [22]. This grading system is more comprehensive than others.
Grade I: NCPAP/non-invasive intermittent PPV/nasal cannula with a flow rate of ≥ 3 L/min and an FiO2 of 0.21; a nasal cannula with a flow rate of 1 L/min–3 L/min, or a hood and an FiO2 of 0.22–0.29; or a nasal cannula with a flow rate of <1 L/min and an FiO2 of 0.22–0.70.
Grade II: Invasive intermittent PPV with an FiO2 of 0.21; NCPAP/ non-invasive intermittent PPV/nasal cannula with a flow rate of ≥ 3 L/min and an FiO2 of 0.22–0.29; a nasal cannula with a flow rate of 1 L/min–3 L/min, or a hood and an FiO2 ≥ 0.30; or a nasal cannula with a flow rate of <1 L/min and an FiO2 of>0.70.
Grade III: Invasive intermittent PPV with a FiO2>0.21; or NCPAP/ non-invasive intermittent PPV/nasal cannula with a flow rate of ≥ 3 L/min with a FiO2 ≥ 0.30 [22].
Jensen-2019: The Jensen-2019 definition of BPD includes four graded categories (0,1,2,3) for infants born <32 weeks GA and evaluation at 36 weeks PMA based on respiratory support status. Grade 0 is no BPD and is on room air. Grade 1 requires nasal cannula (NC) flow ≤ 2 L/min. Grade 2 requires NC flow>2 LPM, CPAP or NIPPV. Grade 3 requires invasive mechanical ventilation [23].
Statistical analysis
Individual patient data (n=220) were used to evaluate the impact of the four varying BPD grading methods on patient-specific disease severity and group/treatment effects. Categorical variables were compared using Pearson’s chi-square or Fisher’s exact tests, as appropriate. Continuous variables were analysed utilizing independent sample t-tests or Mann-Whitney U tests. Comparisons of statistical power and enrollment requirements were developed using SPSS. An alpha level of 0.05 was used to determine significance. Statistical analysis was completed using IBM SPSS Statistics version 28 (IBM Corp., Armonk, NY) and SAS 9.4 (SAS Institute Corp., Cary, NC).
BPD grade assignments for individual patients using each of the four definitions
Shown in Figure 1 are distributions of BPD grades for the entire population of enrolled patients in the data set. Among 220 patients enrolled in the clinical trial analysed, there were 176 survivors. Grade II or “moderate" BPD was significantly different between the four definitions used, with results varying between 44%, 47%, and 17% according to NIH-2001, Jensen-2019 and NIH-2018 definitions, respectively (Figure 1). Additional ‘shifting’ in BPD grades according to these classifications of grade III or “severe” BPD was significantly different at 36%, 36% and 9% according to NIH-2001, NIH-2018 and Jensen-2019 definitions, respectively. Based on the newest definition in Jensen-2019, to be classified as severe BPD, the infant requires mechanical ventilation at 36 PMA.
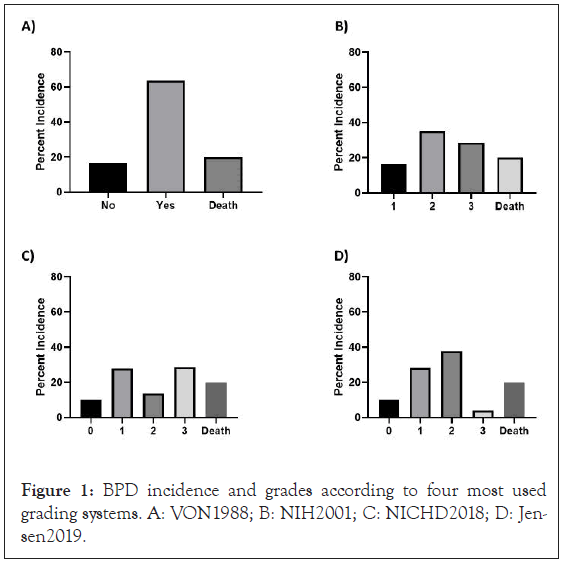
Figure 1: BPD incidence and grades according to four most used grading systems. A: VON1988; B: NIH2001; C: NICHD2018; D: Jensen2019.
Ureaplasma as a contributing factor to BPD risks
All subjects were tested for Ureaplasma positivity by using tracheal aspirates collected at specific time’s points over the course of the trial. DNA was extracted and PCR was conducted for Ureaplasma detection. BPD severity/grading was determined for each patient using the four different BPD scoring systems (VON-1988, NIH-2001, NICHD-2018, or Jensen-2019). PCR analyses were conducted by an external laboratory using standardized methods. Among 220 patients enrolled, there were 76 patients Ureaplasma positive (35% positivity/infectivity rate). Using the VON-1988 and NIH-2001 BPD grading system, Ureaplasma positivity was associated with BPD severity (BPD moderate/severe or death vs. BPD none or mild) (Table 1). In contrast, using the two more recent BPD scoring systems (NICHD-2018, and Jensen-2019), no statistical significance was seen in differences between Ureaplasma positive vs. negative cases (Table 2).
| BPD classification | Group | Ureaplasma | None/Mild | Mod/sev/death |
|---|---|---|---|---|
| VON-1988 | Placebo | Negative | 18.2%# | 0.818 |
| Positive | 4.7%# | 95.3%" | ||
| Azithromycin | Negative | 0.195 | 0.805 | |
| Positive | 0.212 | 78.8%" | ||
| NIH-2001 | Placebo | Negative | 18.5%* | 0.815 |
| Positive | 4.7%* | 95.3%^ | ||
| Azithromycin | Negative | 0.189 | 0.811 | |
| Positive | 0.212 | 78.8%^ | ||
| NICHD-2018 | Placebo | Negative | 0.446 | 0.554 |
| Positive | 0.326 | 0.674 | ||
| Azithromycin | Negative | 0.338 | 0.662 | |
| Positive | 0.333 | 0.667 | ||
| Jensen-2019 | Placebo | Negative | 0.462 | 0.558 |
| Positive | 0.326 | 0.674 | ||
| Azithromycin | Negative | 0.338 | 0.662 | |
| Positive | 0.333 | 0.667 |
Table 2: Association of Ureaplasma positivity with BPD severity and the benefit of azithromycin treatment. #Statistically significant (p=0.044) "Statistically significant (p=0.038) *Statistically significant (p=0.043)^Statistically significant (p=0.037).
Azithromycin treatment benefit based on BPD grading strategy
Table 2 contains the effects of Azithromycin vs. placebo treatment on BPD outcomes. In this original trial, infants enrolled at birth were randomized to receive Azithromycin or placebo for a total of 6 weeks. When the four BPD scoring systems were assigned to each patient in our analysis (VON-1988, NIH-2001, NICHD-2018, and Jensen-2019), two of the systems (VON-1988 and NIH-2001) showed Azithromycin treatment in Ureaplasma positive cases significantly reduced BPD outcomes (Table 1). The incidence of moderate/severe BPD and death was reduced from 95.3% in placebo treated infants to 78.8% in Azithromycin treated infants (Table 2). The statistical conclusions were unaltered if patient deaths were excluded from analyses.
BPD grading strategy impact on statistical power and sample size requirements
In light of the finding about each BPD grading system’s impact on the statistical conclusions of the previously conducted trial of over 220 enrolled preterm infants, we compared sample size requirements at various levels of statistical power using each of the 4 diagnostic strategies (Figure 2). The power of a study is dependent on the effect size or difference between groups and the sample size. The required sample size increases the financial cost of identifying a significant effect. Each BPD grading system has unique criteria for defining or diagnosing BPD. VON-1988 with only two groups and NIH-2001 has a more relaxed criteria in diagnosing BPD, which make it easier to distinguish between groups. They result in higher number of preterm infants diagnosed with BPD, resulting in a higher incidence of BPD. Larger differences between groups increases the statistical power, and high statistical power will require a small sample size. According to our analyses, VON- 1988 and NIH-2001 will produce 0.95 statistical power with 100 enrolled patients (Figure 2), while NICHD-2018 and Jensen-2019 will require more than double the sample size VON-1988 and NIH-2001 required in order to attain the same statistical power. Using NICHD-2018 and Jensen-2019 BPD scoring systems, these will provide a 0.95 statistical power only if there are 225 or 185 enrolled patients respectively (Figure 2).
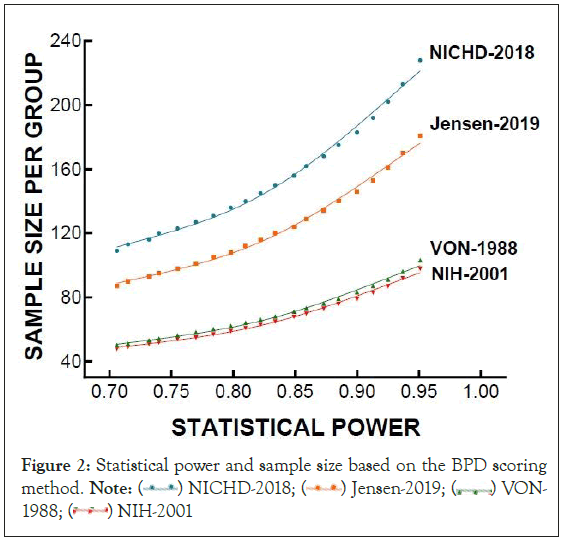
Figure 2: Statistical power and sample size based on the BPD scoring method. Note:  VON-1988;
VON-1988;  NIH-2001
NIH-2001
An important challenge in drug development for preterm neonates has been the steady improvements in survival and therefore, a patient population increasingly immature at birth when enrolled. In the late 1990’s, infants born prior to 26 weeks GA rarely survived whereas many institutions are now successful in survival of patients less than 24 weeks and 400 g at birth. The pathogenesis of BPD, and the clinical definitions of severity, have evolved in recent decades and complicated drug development along with the willingness of study sponsors to invest in the research field. Comparing the four BPD grading strategies, an approximate 2-fold range in number is needed to treat in order to find benefit at equivalent statistical power (using the same exact trial data). We postulate this would be associated with an extended range in clinical research costs, if a trial was developed using the less sensitive grading systems (NICHD-2018, Jensen-2019). Although these issues may be intuitive, this secondary data analysis illustrates the larger impact in the value of establishing a working definition of BPD for clinical trial development and hypothesis driven efforts to address the continued medical problem.
In this study, we analysed one data set from a single clinical trial developed and conducted locally. We investigated and applied the four widely known and most commonly used definitions for BPD (VON-1988, NIH-2001, NICHD-2018, and Jensen-2019) to compare BPD-associated outcomes, severities, and potential treatment benefit of Azithromycin versus placebo, in Ureaplasma positive versus negative cases. The secondary data analysis effort was conducted as a pilot investigation for streamlining guidelines for future clinical trial protocols investigating perinatal infection, macrolide treatment, and respiratory/pulmonary outcomes at The University of Kentucky.
Each of the BPD grading strategies rely on clinical management details of oxygen supplementation and/or use of positive pressure support, with no assessment of pathophysiologic process, laboratory evaluation, or histopathology. The definitions of BPD have changed and evolved over the last few decades; incidence of BPD varied depending upon the definition used. Outcomes, treatment, complications, and prognosis depend on which definition is applied during diagnoses. This is a major impediment for clinical trials investigating BPD mechanisms and/or therapeutic drug development to address this persisting prognosis.
All four definitions diagnose the enrolled infants as having BPD with varying degrees. The main difference was in the severity/grade level of BPD, which was shifting significantly based on each specific definition used. Importantly, grade III or severe BPD shows the most grade shifting according to the definition employed. The NIH-2001 and NICHD-2018 definitions, roughly 36% of the enrolled infants were in the severe BPD grade, in contrast to only 9% of the infants put in this category when the Jensen-2019 was applied. The Jensen-2019 definition put the infant in grade III or severe BPD, only if the infant required mechanical ventilation at 36 weeks’ PMA. Such variation in the classification of severity would impact the approach to therapy used, which can change the outcome of disease progression.
Through this secondary evaluation, we found the statistical conclusions derived from this well-controlled and completed trial were highly dependent on the BPD grading system employed; only two of the four well-recognized scoring guidelines showed infection- and treatment-related differences. Relationship of Ureaplasma infection to BPD severity can be concluded if the VON-1988 or NIH-2001 strategies were used exclusively. Likewise, the benefit of Azithromycin treatment in this study (e.g. with this dosing strategy) is best predicated on the use of the same two grading strategies (VON-1988, NIH-2001). These two scoring definitions are currently in wide use, particularly the VON-1988 system, which compares and benchmarks NICUs from around the world in patient outcomes. Based on the conclusions derived from the VON-1988 grading, rapid screening for Ureaplasma at birth and specific use of Azithromycin would be clearly warranted throughout this network. Additional studies to demonstrate this benefit remain to be conducted, including an ongoing trial in the United Kingdom (AZTEC trial) [24,25]. There is also a need for defining mechanisms involved and treatment strategy optimization.
In summary, the statistical hypotheses tested using this data set with 220 patients, in respect to Ureaplasma impact on BPD outcomes and potential benefit of Azithromycin, were highly dependent on the specific BPD grading system employed. This study illustrates how important BPD grading is for defining causative mechanisms and therapeutic strategies. A consensus in defining BPD from a clinical perspective would facilitate opportunities for reactive and proactive strategies. Clear agreement on the most accurate BPD grading system across the NICU network can also enhance prediction of patient pulmonary care needs at discharge and follow-up, along with improvement of parental understanding in long-term outcomes.
MA carried out the secondary study and contributed to writing the manuscript. YA contributed manuscript writing and results interpretation. AS did the statistical analysis. HH and BS acquired data from the original clinical trial, and reviewed manuscript. KW reviewed the manuscript. HB and JB conceived the study design, interpretation of results, and edited the manuscript. All authors have read and approved the final version of this manuscript.
This work was supported in part by the University of Kentucky Maternal and Pediatric Research Alliance, the Regina Drury Endowment Pediatric Research Fund, and the Kentucky Children’s Hospital Office of Pediatric Research.
The co-authors appreciate the extensive support provided by clinical research and bedside clinical staff involved in the original clinical trial protocols and data management strategies that have made this secondary effort possible.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ajour M, Alsiraj Y, Schadler A, Huang H, Schanbacher B, Williams K, et al. (2023) Ureaplasma, Azithromycin, and Varying Definitions of BPD (Bronchopulmonary Dysplasia) in Preterm Infants: Challenges for Clinical Care and Drug Development. J Clin Trials. 13:536.
Received: 16-May-2023, Manuscript No. JCTR-23-24945; Editor assigned: 18-May-2023, Pre QC No. JCTR-23-24945(PQ); Reviewed: 01-Jun-2023, QC No. JCTR-23-24945; Revised: 08-Jun-2023, Manuscript No. JCTR-23-24945(R); Published: 15-Jun-2023 , DOI: 10.35248/2167-0870.23.13.536
Copyright: © 2023 Ajour M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.