
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Case Report - (2024)Volume 10, Issue 3
A group of COVID-19 patients develops severe or critical disease accompanied by the cytokine storm syndrome. Cytokines are essential to the pathophysiology of disease and Interleukin 6 (IL-6), Interleukin 1 (IL-1) and Tumor Necrosis Factor Alpha (TNF-α) appear to be harmful, particularly in the context of the cytokine release syndrome. CD6 is a membrane glycoprotein expressed primarily in mature, activated T-cells. Ligand binding of CD6, increases events such as adhesion, activation, proliferation, differentiation and survival. In addition, CD6 mediates interaction between T-cells and antigen-presenting cells, contributing to the maturation of immune synapses. CD6-mediated costimulation contributes to the maturation of a Th1 pattern in human T-cells and preferentially promotes a proinflammatory response characterized by the secretion of TNF-α, IL-6 and interferon.
Partial pressure of oxygen; Interleukin; Immune synapses
Itolizumab is a humanized monoclonal antibody developed at the center of molecular immunology, in Cuba that recognizes a region in the distal domain of the human CD6. The effect is associated with the reduction of the secretion of proinflammatory cytokines including interferon- γ IL-6 and TNF-α [1]. The antibody has demonstrated to be safe and efficacious in patients with moderate to severe psoriasis.
Studies from blood and tissue samples from patients with severe psoriasis showed that itolizumab reduces the proliferation of Tcells and serum concentration of IL-6, TNF-α and interferon- γ. On account of its well proven effect on T-cell activation and proinflammatory cytokines production, itolizumab was included as part of an expanded access protocol in severe and critically ill COVID-19 patients in Cuba. The study is ongoing and here we describe the outcome of the first three patients receiving the antibody.
Patient 1
A 53-year-old woman with a personal history of essential hypertension and type 2 diabetes mellitus presented with symptoms of polypnea of more than 40 rpm, use of respiratory ancillary musculature and dry cough. She arrived from a foreign country where COVID-19 was spreading. Symptoms started on March 23 and COVID-19 was diagnosed on March 26 on the basis of positive RT-PCR for SARS-CoV-2. Initial gasometry showed moderate hypoxemia and respiratory alkalosis with a PO2/FiO2 ratio of 191. Chest X rays showed interstitial lesions in both lung fields [2]. She started therapy with lopinavir/ ritonavir, chloroquine, recombinant IFN alpha-2b and rocephin. In spite of treatment, the illness subsequently progressed to hypoxemic respiratory failure warranting the initiation of invasive mechanical ventilation.
At day 13 of her admission in the ICU, she showed radiologic worsening of the interstitial multifocal pneumonia, with elevation of alkaline phosphatase, Lactate Dehydrogenase (LDH), erythrocyte sedimentation rate and D-Dimer. Physicians administered itolizumab at a dose of 200 mg [3]. After 48 hrs of the first itolizumab dose, PO2/FiO2 improved and there were evidences of radiological improvement. Patient was extubated after the first dose of the antibody and her status changed from critical to severe. She received a second dose of the antibody (200 mg), 48 hrs after the first infusion. Three days after the first administration, patient was hemodynamically stable and has spontaneous ventilation. Interleukin 6 levels were evaluated before itolizumab administration and after 2 and 7 days of the first administration. Interleukin 6 levels reduced overtime from 172 pg/ml to 60 pg/ml (day 7) as depicted. Interleukin 1 was evaluated at the same time intervals, but it was undetectable. In addition, Aspartate Amino Transferase (AST) concentrations were evaluated at different time points showing a reduction from 43 U/L to 24 U/L after 7 days. No adverse events related with itolizumab were reported [4].
Patient 2
An 89-year-old man with a personal history of chronic ischemic cardiopathy, permanent atrial fibrillation, hypertension and hypothyroidism. He has a previous history of alcoholism and several hospital admissions in the last three months on account of infectious respiratory diseases. He came to the hospital on April 2nd, after 7 days of shortness of breath, fever, asthenia and dry cough. He was in contact with a person who returned from a foreign country, where COVID-19 was extending. Physical examination shows signs of respiratory failure characterized by tachycardia, polypnea, intercostal and supraclavicular muscle retraction, high blood pressure, oxygen saturation of 82%, poor diuresis and drowsiness [5]. Chest X rays showed bilateral pulmonary inflammatory infiltrates, predominantly in the right lung. Admission electrocardiogram showed an atrial fibrillation with rapid ventricular response and the initial gasometry showed severe hypoxemia and respiratory alkalosis. Patient also had leukocytosis, altered globular sedimentation rate as well as elevated values of amino-aspartate transaminase, LDH, Ddimer and positive C-reactive protein. He was admitted into the ICU requiring invasive mechanical ventilation. Treatment with lopinavir-ritonavir, chloroquine, interferon alpha-2b, meropenem and linezolid was initiated. Three days after his admission into the ICU, itolizumab was prescribed, due to worsening of the bilateral pulmonary infiltrates together with a deterioration of the ventilatory function (PO2/FiO2=173). After the first antibody infusion, PO2/FiO2 significantly increased (PO2/FiO2=320) and there were evidences of radiological improvement. Three days after, patient showed radiological worsening of the left lung, characterized by alveolar hypoventilation and atelectasis; then, a 70 % pneumothorax was established. Treatment of the pneumothorax with minimal pleurotomy was very demanding and required 3 days for the resolution.
The patient received a second infusion of the monoclonal antibody 72 hours after the first, while a third dose was administered at the discretion of the treating physicians, two days after the second. In total, patient received three doses of itolizumab (200 mg) without any related adverse event.
Interleukin 6 levels were evaluated before itolizumab administration and after 2, 4 and 7 days of the first administration. Interleukin 6 was extremely high at baseline (623 pg/ml) and even though cytokine levels reduced roughly 50%, the lowest value remained above 300 pg/ml after 7 days. Interleukin 6 kinetics. Apart from interleukin 6, interleukin 1 was undetectable at this time point of the disease. Interesting, in the case of AST, a significant reduction is detecting at day 7 (Pretreatment: 156U/L, D7: 40 U/L). After 10 days of admission into the ICU, patient presented a myocardial dysfunction and shock that required vasoactive support with norepinephrine. On day 13, he finally died, on account of a mixed cardiovascular and respiratory failure.
Patient 3
An 81 years old female, who’s COVID-19 positive contact was not identified at the moment of hospitalization. She started symptoms on April 2nd and entered the ICU on April 5th. Patient has a previous history of hypertension, diabetes mellitus, glaucoma and smoking habit. She was admitted with frequent cough, wheezing and diarrhea. The diagnosis of viral pneumonia by COVID-19 was confirmed by RT-PCR on April 7th. Oxygen support at 5 L/min and treatment with ceftriaxone, lopinavir-ritonavir, chloroquine and interferon alpha-2b was indicated. Chest images showed bilateral interstitial infiltration in both lungs. Patient condition was classified as severe, although she did not require invasive mechanical ventilation. A dose of itolizumab (200 mg) was given the day after her admission into the ICU. Two days after the antibody administration, together with the rest of the therapy, there was an improvement of the respiratory distress while the chest image showed a decrease of the alveolar interstitial infiltrate of both lungs. Patient leaved the ICU after a favorable clinical and radiological evolution. Interleukin 6 concentration was measured prior and after the antibody administration. IL-6 level at baseline was lower than in previous cases (30 pg/ml), but also decreased 48 hrs and 168 hrs after itolizumab infusion, in parallel with patient recovery. Interleukin 1 and TNF-α were untraceable before and 48 hrs after the antibody administration. Regarding to AST concentration, the values were in the normal range during the treatment period (Figure 1) [5].
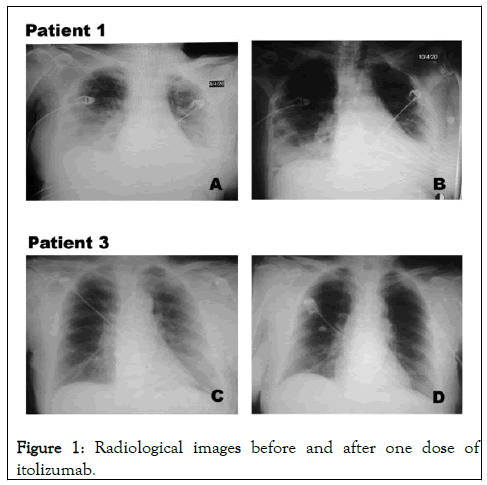
Figure 1: Radiological images before and after one dose of itolizumab.
Three patients with diagnosis of COVID-19 classified as critically ill (2) and severely ill (1) were included in an expanded access trial to receive itolizumab in addition to standard treatment. The study was approved by a central ethical review board, created especially for COVID-19 and by the Cuban Regulatory Agency (CECMED). Before the treatment, informed consent was obtained from enrolled patient.
Laboratory results included blood routine, leucocyte subsets and blood biochemical parameters were collected. The level of inflammatory cytokines in serum was measured using human quantikine ELISA Kits from R and D systems (Minneapolis, USA): HUMAN IL-6 quantikine ELISA Kit (Cat# S6050). Human IL-1 beta/IL-1F2 quantikine ELISA Kit (Cat# SLB50). Human TNF-α quantikine ELISA Kit (Cat# STA00D).
Interleukin-6 levels were noted to be elevated in SARS-CoV2 patients in correlation with disease severity. As a result, several anti-cytokine therapies have been tried for treating the hyperinflammatory phase of COVID-19. Here we report the use of a well-known anti-inflammatory antibody targeting CD6 to treat the cytokine release syndrome arising in COVID-19 patients. Using an anti-CD6 antibody could reduce the concentration of several pro-inflammatory cytokines, including IL-6, interferon- γ, TNF-α and interleukin 17, among others, representing an advantage as compared to single-cytokine targeting antibodies. The antibody would not exacerbate lymphopenia since it does not induce complement or antibody dependent cytotoxicity [6].
These three SARS-CoV2 patients developed severe respiratory distress together with multifocal interstitial pneumonia. Two patients required invasive mechanical ventilation while the third patient only needed oxygen supply. They all received the anti- CD6 antibody itolizumab in combination with other drugs incorporated into the Cuban national protocol, including lopinavir/ritonavir, chloroquine and interferon alpha-2b. Regarding to laboratory parameters, AST is strongly associated with mortality risk compared to other parameters, reflecting liver and kidney injury. In our cases, critically ill patients showed a significant reduction of AST concentration after the treatment suggesting an improvement in the function of these organs (Figure 2).
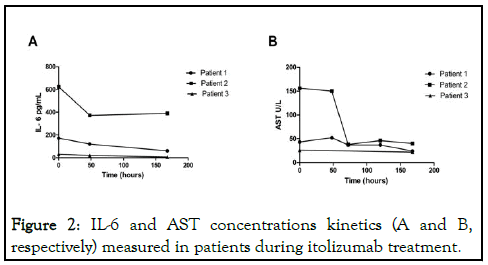
Figure 2: IL-6 and AST concentrations kinetics (A and B, respectively) measured in patients during itolizumab treatment.
Itolizumab was very safe and did not seem to exacerbate opportunistic secondary infections. Our preliminary findings support that interleukin 6 levels correlated with the severity of the disease and that the antibody was capable of reducing IL-6 concentration in all three subjects. Unfortunately, in spite of the initial clinical and radiological favorable response, one patient died after subsequent respiratory and cardiovascular complications. In this particular case, IL-6 concentration was extremely elevated at the moment of itolizumab infusion and presumably the consequences of the hyperinflammatory syndrome (thrombosis, alveolar damage and severe tissue hypoxia) were irreversible at the moment of treatment [8].
In summary, in these severe and critically-ill patients, itolizumab was able to reduce IL-6 concentrations. All patients showed rapid ventilatory and radiological improvement and notably, itolizumab-related adverse events were not reported. We anticipate that the timely use of this anti-inflammatory antibody in combination with the appropriate anti-viral and anticoagulant therapy could reduce the mortality associated with COVID-19. The analysis of the complete-series is pending to be published.
We are extremely grateful to all ICU physicians, nurses and general staff working with severe and critically ill COVID19 patients.
Seven authors (GL, MC, MR, DS, ZM, KL and TC) currently work for the center of molecular immunology, the institution that generated and originally patented itolizumab. The remaining authors do not have any commercial or financial relationships that could be taken as a potential conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Filgueira LM, Cervantes JB, Lovelle1 OA, Herrera C, Figueredo C, Caballero JA, et al. (2024) Use of an Anti-CD6 Antibody (Itolizumab) for the Treatment of COVID-19 Patients with Cytokine Release Syndrome Report of Three Cases. Immunotherapy. 10:257.
Received: 14-Aug-2020, Manuscript No. imt-24-5999; Editor assigned: 20-Aug-2019, Pre QC No. imt-24-5999 (PQ); Reviewed: 03-Sep-2020, QC No. imt-24-5999; Revised: 01-Jun-2024, Manuscript No. imt-24-5999 (R); Published: 28-Jun-2024 , DOI: 10.35248/2471-9552.24.10.257
Copyright: © 2024 Filgueira LM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.