Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Case Report - (2021)
Choroideremia is a rare, X-linked chorioretinal dystrophy caused by mutations in the CHM gene. Diagnosis is suggested by the characteristic phenotype, X-linked family history and level of Rab Escort Protein 1 (REP1) in peripheral blood mononuclear cells. Genetic testing is essential for confirmation. This case describes the use of multimodal imaging for early diagnosis of choroideremia in an asymptomatic young adult. A 27-year-old asymptomatic male was referred to the retina clinic at Louisiana State University Health Care Network in March 2020 for incidental findings. He did not have any visual complaints. His clinical examination was subtle relative to the classic extensive chorioretinal atrophy with scleral exposure typically seen on fundus examination and color fundus photography in choroideremia. Multimodal imaging findings prompted genetic testing leading to an early diagnosis of choroideremia. Multimodal imaging helps understand the natural history and improved evaluation and diagnosis of rare conditions.
Choroideremia; Multimodal imaging; CHM gene; Rab escort protein (REP)
Choroideremia is a rare, X-linked chorioretinal dystrophy caused by mutations in the CHM gene. These mutations either cause a loss of function or absence of the Rab Escort Protein 1 (REP1) that is important for the prenylation process and intracellular transport of the REP1.This results in progressive degeneration of the outer retina, Retinal Pigment Epithelium (RPE), photoreceptor layer and the inner choroid [1].
Affected males typically present with nyctalopia in childhood that evolves into severe peripheral visual field loss and eventually central vision loss later in life. Diagnosis is suggested by the characteristic phenotype, X-linked family history and level of REP1 in peripheral blood mononuclear cells. Genetic testing is essential for confirmation [2].
The fundus initially demonstrates peripheral pigmentary changes that progress to distinct chorioretinal atrophy beginning near the equator and extending centripetally towards the anterior retina and posterior pole [3]. The chorioretinal atrophy in later stages is so severe that the sclera and the large choroidal vessels may be exposed. Fundus Auto fluorescence (FAF) usually illustrates a pattern of regional hypo-autofluorescence corresponding to atrophic areas abutting sharply delimited borders of hyper-autofluorescence corresponding to residual retinal tissue. Optical Coherence Tomography (OCT) may show RPE and Ellipsoid Zone (EZ) disruption, decrease in outer nuclear layer thickness and appearance of outer retinal tubulations. In advanced cases, there may be variable cystoid macular edema from RPE dysfunction and rarely, formation of macular hole. OCT angiography (OCTA) shows a decreased choriocapillaris density primarily in areas underlying retinal and RPE loss [4].
The present case describes the use of multimodal imaging for early diagnosis of choroideremia in an asymptomatic young adult.
A 27-year-old asymptomatic male was referred to the retina clinic at Louisiana State University Health Care Network in March 2020 for an unusual appearance of fundus from an optometry clinic where he went for a routine examination. He was asymptomatic and denied history of nytalopia and/or difficulty driving at night. There was no family history of hereditary retinal diseases.
His best-corrected visual acuity was 20/20 OD and 20/25 OS. Intraocular pressures were 18 mmHg OD and 17 mmHg OS. Slit lamp bio microscopy was unremarkable without inflammation in the anterior chamber or vitreous OU. Dilated fundus examination and wide-field color fundus photography were notable for attenuated vessels and macular, mid-peripheral and far peripheral pigmentary changes OU. FAF depicted multiple areas of hypo-auto fluorescence particularly in the peripapillary and macular area sparing the fovea OU consistent with RPE degeneration. A 24-2 Humphrey visual field demonstrated an enlarged blind spot OU. OCT was remarkable for diffuse RPE and EZ disruption with sub-foveal sparing OU. OCTA showed areas of decreased choriocapillaris density/nonperfusion OU (Figures 1a-1h).
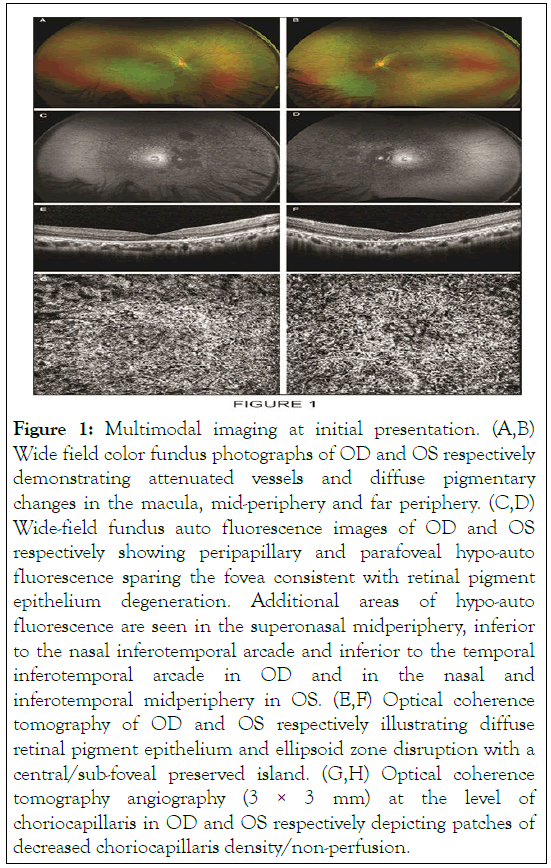
Figure 1: Multimodal imaging at initial presentation. (A,B) Wide field color fundus photographs of OD and OS respectively demonstrating attenuated vessels and diffuse pigmentary changes in the macula, mid-periphery and far periphery. (C,D) Wide-field fundus auto fluorescence images of OD and OS respectively showing peripapillary and parafoveal hypo-auto fluorescence sparing the fovea consistent with retinal pigment epithelium degeneration. Additional areas of hypo-auto fluorescence are seen in the superonasal midperiphery, inferior to the nasal inferotemporal arcade and inferior to the temporal inferotemporal arcade in OD and in the nasal and inferotemporal midperiphery in OS. (E,F) Optical coherence tomography of OD and OS respectively illustrating diffuse retinal pigment epithelium and ellipsoid zone disruption with a central/sub-foveal preserved island. (G,H) Optical coherence tomography angiography (3 × 3 mm) at the level of choriocapillaris in OD and OS respectively depicting patches of decreased choriocapillaris density/non-perfusion.
An infectious workup including testing for tuberculosis, syphilis, Lyme disease and human immunodeficiency virus was negative. Inflammatory work-up including testing for antinuclear antibodies, sarcoidosis and ankylosing spondylitis was also negative. Given the findings on multimodal imaging, genetic testing with Spark therapeutics was offered to the patient. This revealed a mutation in CHM gene at exon 5; a frameshift deletion that yields a premature translational stop codon previously identified in the literature [5]. This was consistent with a diagnosis of choroideremia. An extensive discussion and counseling regarding the natural history of choroideremia was done and resources on the ongoing clinical trials for this condition were provided to the patient.
At one year follow up in March 2021, his best-corrected visual acuity was stable at 20/20 OD and 20/25 OS. Dilated fundus examination and wide-field color fundus photography were notable for persistent macular, mid-peripheral and far peripheral pigmentary changes OU. FAF depicted an interval increase in multiple areas of patchy hypo-auto fluorescence OU. OCT was stable from prior depicting diffuse RPE and EZ disruption with sub-foveal sparing OU. OCTA showed an interval increase in areas of decreased choriocapillaris density/non-perfusion OU (Figures 2a-2d) .
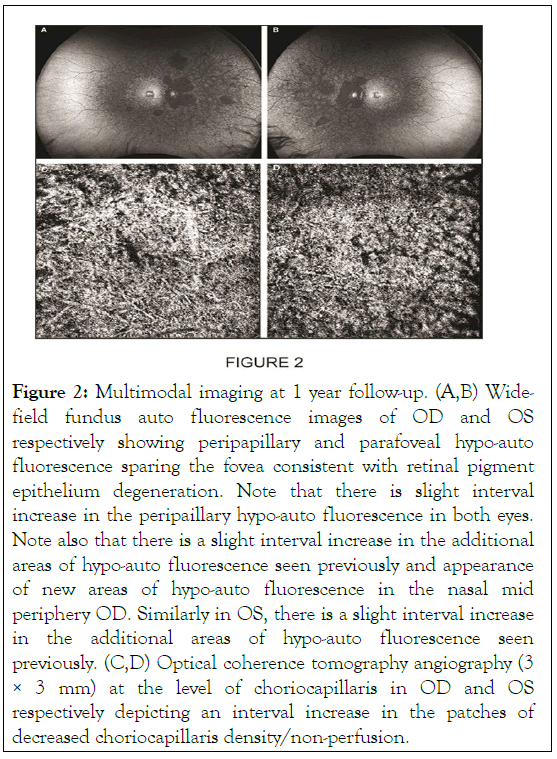
Figure 2: Multimodal imaging at 1 year follow-up. (A,B) Widefield fundus auto fluorescence images of OD and OS respectively showing peripapillary and parafoveal hypo-auto fluorescence sparing the fovea consistent with retinal pigment epithelium degeneration. Note that there is slight interval increase in the peripaillary hypo-auto fluorescence in both eyes. Note also that there is a slight interval increase in the additional areas of hypo-auto fluorescence seen previously and appearance of new areas of hypo-auto fluorescence in the nasal mid periphery OD. Similarly in OS, there is a slight interval increase in the additional areas of hypo-auto fluorescence seen previously. (C,D) Optical coherence tomography angiography (3 × 3 mm) at the level of choriocapillaris in OD and OS respectively depicting an interval increase in the patches of decreased choriocapillaris density/non-perfusion.
In this report, the authors describe the use of multimodal imaging in a case of choroideremia presenting early in the disease course in an asymptomatic adult male. Typically, affected males generally display nyctalopia in childhood progressing to severe peripheral visual field loss and eventual central vision loss by the fifth or sixth decade of life. This 27-year-old patient presented without any visual complaints and was referred to the retina clinic for incidental findings. His clinical examination was subtle relative to the classic extensive chorioretinal atrophy with scleral exposure typically seen on fundus examination and color fundus photography in this condition. Multimodal imaging findings prompted genetic testing leading to an early diagnosis of choroideremia [1].
At present, there is no Food and Drug Administration (FDA) approved treatments for choroideremia. Several approaches are under investigation including gene therapy, stem cell implantation and retinal prosthesis system in advanced cases. The most promising treatment currently in clinical trials is a gene therapy approach using an Adeno-Associated Vector (AAV) surgically delivered in the sub retinal space. Expression of CHM gene encoding a functional REP1 is reasonably expected to provide therapeutic benefit. Early intervention may likely maximize the benefit of a gene therapy approach by increasing the production of functional REP1. This could slow and/or ideally halt the progressive chorioretinal degeneration characteristic of choroideremia. Other possible future therapeutic modalities include nonsense suppression therapy and optogenetic therapy [3,6].
The present case describes the use of multimodal imaging in early choroideremia that prompted genetic testing to confirm the diagnosis in an asymptomatic young male. Multimodal imaging helps understand the natural history and improved evaluation and diagnosis of rare conditions.
Citation: Contreary C, Reinoso M (2021) Use of Multimodal Imaging for Early Diagnosis of Choroideremia in an Asymptomatic Young Adult. J Clin Exp Ophthalmol. S18:001
Received: 03-Aug-2021 Accepted: 17-Aug-2021 Published: 24-Aug-2021 , DOI: 10.35248/2155-9570.21.s18.001
Copyright: © 2021 Contreary C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.