Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2024)Volume 14, Issue 2
Pulmonary Embolism (PE) is a serious medical condition that presents diagnostic challenges due to its non-specific signs and symptoms. In the pathophysiology of PE, pulmonary vascular artery obstructions can cause a range of symptoms, from mild to severe, depending on the size and location of the obstruction. Unfortunately, current diagnostic tests for PE, such as Computed Tomography Pulmonary Angiogram (CTPA) and plasma D-dimer measurement, are inefficient and costly. However, hypoxia and hypocapnia are two hallmark signs of PE, which manifest as decreased oxygen levels and increased carbon dioxide levels, respectively. Therefore, measuring End-Tidal CO2 (ETCO2) levels, which indicate the level of Carbon Dioxide (CO2) in expiration, is a feasible and straightforward method for diagnosing PE.
We conducted a study with 479 patients to investigate the relationship between ETCO2, D-dimer and CTPA. Our findings suggest that ETCO2 measurement is sensitive enough to exclude PE in approximately 82% of cases. Based on this data, we propose a machine-learning tool for predicting PE using ETCO2 measurements. This tool has the potential to be developed into an automated diagnostic aid for PE. In conclusion, our study highlights the potential of ETCO2 measurement as a diagnostic tool for PE. With further development and refinement, an automated diagnostic tool using ETCO2 measurements could provide a more efficient and cost- effective means of diagnosing PE, which would benefit patients and healthcare providers alike.
Pulmonary embolism; Machine learning; Computed tomography
PE: Pulmonary Embolism; MI: Myocardial Infarction; CVA: Cerebrovascular Accidents; VTE: Venous Thromboembolism; NICE: National Institute for Health and Care Excellence; RV: Right Ventricle; CTPA: Computed Tomography Pulmonary Angiogram; AMAU: Acute Medical Assessment Unit; ROCs: Receiver Operating Curves; AUC: Area Under the Curve; NPV: Negative Predictive Value; PPV: Positive Predictive Value; CT: Computed Tomography
Pulmonary Embolism (PE) is a widespread cardiovascular and cardiopulmonary ailment. It occurs when deep venous thrombi detach and embolize in the pulmonary circulation, leading to impaired gas exchange and circulation due to pulmonary vascular occlusion. Notably, PE has been identified as the third most prevalent cause of death globally and it may consequently cause cardiovascular injuries such as Myocardial Infarction (MI) and Cerebrovascular Accidents (CVA) [1].
Venous Thromboembolism (VTE) is a potentially serious medical condition that commonly affects the lower extremities or pelvic veins and can lead to clinically significant Pulmonary Embolism (PE). However, thromboembolic events occurring in the upper extremities have also been shown to result in PE. VTE that is not treated in a timely and appropriate manner can have a mortality rate of up to 25%, which can be significantly reduced to 1-5% with prompt treatment. Therefore, it is imperative that PE is diagnosed at critical stages to ensure the best possible care is provided [2]. As per the data shared by The National Institute for Health and Care Excellence (NICE), the annual incidence of Venous Thromboembolism (VTE) is approximately 2 in 1000 persons, whereas the yearly incidence of Pulmonary Embolism (PE) is around 7-8 per 10,000 persons in the United Kingdom [3]. In the United States, Pulmonary Embolism (PE) is responsible for causing approximately 100,000 to 200,000 deaths every year. Patients with PE can experience a wide range of clinical symptoms or even be asymptomatic [4]. Pulmonary Embolism (PE) is a potentially life-threatening medical condition that occurs when a blood clot travels to the lungs. A number of risk factors have been identified for PE, several of which are modifiable. Notably, obesity, cigarette smoking and hypertension have been identified as the most common reversible risk factors associated with PE. However, PE has also been linked to a range of other factors, including surgery, trauma, cancer, oral contraceptives, pregnancy and postmenopausal hormone replacement therapy. In addition, complex medical conditions such as pneumonia and congestive heart failure have also been associated with an increased risk of developing PE. Given the serious nature of this condition, it is important to be aware of the various risk factors and to take appropriate steps to mitigate them whenever possible. Numerous reports have highlighted a genetic predisposition to venous thrombosis. Twin studies have reaffirmed the significant role of an inherited prothrombotic state in this regard. The pathophysiology of Pulmonary Embolism (PE) can lead to hemodynamic compromise, particularly if the clot burden is high. Several pathological disturbances have been linked to PE, with the influence on the response of the Right Ventricle (RV) being the most crucial determinant of PE patient outcomes [5].
The lungs receive systemic circulation via the bronchial circulation, which subsequently drains into the pulmonary vein. This left-to-left anatomic shunt leads to a minor decline in arterial Oxygen Pressure (PaO2) from 100 mmHg in the pulmonary capillaries to 95 mmHg in the pulmonary vein. The Alveolar-arterial gradient (A-a) measures the drop in oxygen partial pressure from the Alveoli (PaO2) to the pulmonary vein (PaO2) due to shunting, which might exacerbate in certain pathological conditions. In addition, right-to-left shunts may cause further reduction in PaO2, resulting in hypoxemia and an elevated A-a gradient. Furthermore, diffusion problems could cause an increased A-a gradient as arterial blood may not reach equilibrium with alveolar air due to limited gas exchange diffusion [6].
The clinical assessment of a patient with suspected Pulmonary Embolism (PE) is largely dependent on the probability of PE and the patient’s overall stability, given the variability in symptomatology. To aid clinicians in evaluating the likelihood of PE and thromboembolic events, several scoring systems have been developed. Examples of such systems include the Wells criteria and Geneva score. These systems provide a standardized approach to assist clinicians in assessing the likelihood of PE and are widely used in clinical settings. By utilizing these scoring systems, clinicians can more confidently evaluate patients with suspected PE and initiate appropriate treatment plans, improving patient outcomes [7]. During resuscitation, a low EtCO2/ PaCO2 ratio may indicate pulmonary embolism. In a study, EtCO2 levels were significantly lower in cardiac arrest caused by pulmonary embolism than by primary arrhythmia, hypoxia and hyperkalaemia. PaCO2 levels were higher in cardiac arrest caused by pulmonary embolism than in the other causes of cardiac arrest [8]. Validated clinical prediction rules are a useful tool in the diagnosis of Pulmonary Embolism (PE) as they assist in estimating the pre-test probability in patients for whom acute PE is being considered. In addition to these rules, D-dimer tests and imaging studies can be employed for diagnosis. However, in patients who demonstrate a low pre-test probability of PE and meet all pulmonary embolism rule-out criteria, these practices may not be essential. Furthermore, it has been argued that using imaging studies as an initial test in patients with low or intermediate pre-test probability of PE is not a recommended practice. Nevertheless, patients presenting with a high pre-test probability of PE should undergo imaging studies using CT Pulmonary Angiography (CTPA).
In cases where CTPA is unavailable or contraindicated in patients, it is recommended to opt for Ventilation-Perfusion (V/Q) scans. These scans can serve as a useful alternative to CTPA in such circumstances. It is imperative to note that V/Q scans are not as sensitive or specific as CTPA and hence, should be used judiciously in the absence of CTPA. However, V/Q scans can still provide valuable diagnostic information in cases where CTPA is not feasible [9].
Respiratory dead space refers to the lung units that are ventilated but do not contribute to gas exchange due to the absence of direct contact between the expired gas from these units and pulmonary capillary blood flow. This phenomenon can be further classified into anatomical dead space, also known as airway dead space and alveolar dead space. Anatomical dead space, as the name implies, refers to the portion of the respiratory system that does not participate in gas exchange, such as the trachea, bronchi and bronchioles. On the other hand, alveolar dead space is the portion of the respiratory system that does not participate in gas exchange due to the presence of ventilation-perfusion mismatch in the alveoli [10].
Capnography is a non-invasive technique that measures the endtidal partial pressure of carbon dioxide in expired gas, providing valuable information on the metabolism of both intubated and spontaneously breathing patients, as well as ventilation and perfusion. This method is widely used in both clinical and research settings to monitor respiratory function, assess the efficacy of interventions and diagnose and manage a range of conditions, including respiratory distress, hypoxia and cardiac arrest. Capnography offers numerous benefits over other methods of respiratory monitoring, including ease of use, portability and real-time feedback, making it an essential tool for healthcare providers and researchers alike [11].
Capnometry, a method used to measure the amount of carbon dioxide expelled during anesthesia, has been in use in the medical field since the 1950s. Despite its early introduction, the method was not widely implemented until the 1980s. With the advent of smaller machines, capnometry finally entered the anaesthesia field, allowing for more precise monitoring of patients [12]. Capnography has shown itself to be a versatile tool with numerous applications in the field of prehospital assessment and triage. It has been utilized in emergency departments and critical care units to monitor patients experiencing cardiac arrest and undergoing procedural sedation. Additionally, capnography has found use as an effective means of diagnosing victims of chemical terrorism. Its utility in these contexts is due to its ability to provide reliable and immediate feedback on a patient’s respiratory and circulatory systems, enabling medical professionals to make informed decisions regarding patient care [11]. Capnography, a medical diagnostic tool that measures carbon dioxide levels in exhaled air, can be categorized into two types: qualitative and quantitative. Qualitative capnography utilizes a colourimetric device that changes colour in response to the amount of CO2 present in the sample. In contrast, quantitative capnography provides a numerical value of end-tidal CO2 and often includes a waveform plotted against time. Capnography has proven useful in a variety of medical conditions, including metabolic acidaemia, procedural sedation, mechanically ventilated patients and cardiac arrest. Additionally, it may have applications in the management of trauma, seizure and respiratory conditions such as pulmonary embolism, although further studies are required to confirm its efficacy in these contexts [11].
ETCO2 also known as the partial pressure of carbon dioxide at the end of an exhaled breath, is a vital parameter for assessing pulmonary circulation and ventilation. The ETCO2 measurement can be used as a substitute for dead space ventilation, which is a significant factor in the assessment of pulmonary embolism. By evaluating the ETCO2 levels, clinicians can gain insight into the extent of ventilation-perfusion mismatch, which can help in the diagnosis and management of pulmonary embolism. Therefore, ETCO2 monitoring is an essential tool for clinicians dealing with respiratory issues [13]. A study of 298 patients suspected of having PE found that those diagnosed with PE had lower ETCO2 levels than healthy individuals. Capnography combined with a wells score of less than 4 increased the negative predictive value to 97.6% [13]. Capnography has limitations, including its limited use in certain conditions and with certain patients, as well as the need for clinical assessment when using this method [11].
The present study aims to evaluate the efficacy of using ETCO2 measurement as a screening tool for Pulmonary Embolism (PE) in 100 patients over a period of six months in 2012. The study obtained data on demographic information, wells’ score, D-dimer levels, symptoms and Computed Tomography Pulmonary Angiogram (CTPA) results, which served as the gold standard. All patients underwent ETCO2 measurements within 24 hours of presentation. Of the 100 patients, 38% were ultimately diagnosed with PE. The results of the study indicated that the average ETCO2 value in patients with a positive CTPA result was 25.13 mmHg (SD 3.75) with a range of 18-31.5 mmHg. Conversely, the average ETCO2 value in patients negative for PE was 33 mmHg (SD 8.25) with a range of 9.75-49.5 mmHg. Interestingly, all patients who tested positive for PE had an ETCO2 value of less than 32.3 mmHg, which had 100% sensitivity and 68% specificity, a negative predictive value of 100% and a positive predictive value of 66%. These findings clearly demonstrate the effectiveness and reliability of ETCO2 measurements as a preliminary screening tool for patients with suspected PE. Using ETCO2 measurements in conjunction with D-dimer tests can reduce the number of patients who require CTPA. Specifically, patients with a negative ETCO2 test result and a low D-dimer level can be safely excluded from further testing.
This cohort study aimed to evaluate the efficacy of ETCO2 measurement as a diagnostic tool for Pulmonary Embolism (PE) in comparison with Computed Tomography Pulmonary Angiography (CTPA). The study included 487 patients suspected of having PE and ETCO2 was measured before the CTPA report was obtained, to ensure unbiased results. The sensitivity and predictivity of ETCO2 were compared with those of CTPA to determine its effectiveness in diagnosing PE.
Ethical approval
Ethical approval was granted by Bradford Leeds Research Ethics Committee (REC number: 17/YH/00), Bradford Teaching Hospitals, NHS Foundation.
A prospective study was conducted at Bradford Teaching Hospital in Bradford, United Kingdom, to investigate the potential of ETCO2 as a screening tool for diagnosing Pulmonary Embolism (PE). The study’s patient sample comprised both inpatients and patients admitted to the Acute Medical Assessment Unit (AMAU) who were suspected of having a PE, as determined by either a high wells’ score or a positive plasma D-dimer. The exclusion criteria included non-invasive ventilation, pregnancy, inability to consent, known type 2 respiratory failure, oxygen therapy greater than 4 l/min, as well as neuromuscular disorders. Data was collected from patients twice, once during a 6-month period in 2012 and again during an eight-month period in 2019.
In cases where patients displayed high suspicion of Pulmonary Embolism (PE), CTPA scans were performed without the determination of D-dimer. Daily communication was maintained with the radiology department to identify patients who had requested a CTPA scan. The results of the CTPA scan were considered the standard for the diagnosis of PE. Patients who had undergone a CTPA scan were approached for consent to determine ETCO2 levels within 24 hours of symptom onset. Out of the 582 patients screened, 35 were excluded as their ETCO2 levels were obtained 24 hours’ post-symptom onset and 68 individuals were excluded due to inadequate consent. No patient was enrolled multiple times.
Patient demographic data was obtained from medical records, along with a detailed medical history that included information on smoking status and co-morbidities. Additionally, the Well’s score was obtained for each patient as assessed by the admitting physician. Plasma D-dimer test results were also recorded if requested by the patient’s physician. It is important to note that this study did not interfere with the management of the patient group. Upon receipt of informed consent, a qualified researcher, who was blinded to the diagnosis, conducted ETCO2 measurements. The Nellcor N85 handheld capnograph/pulse oximeter and CapnostreamTM 35 portable respiratory monitor PM35MN with Microstream ETCO2 and NllcorTM SpO2 Medtronic were used to record the ETCO2 values. To ensure accuracy, five capnographs were employed, which were calibrated to zero and 5.6% of CO2 and verified by the medical physics department at the Bradford Royal Infirmary. The ETCO2 measurements were taken using plastic tubing consisting of an uptake mouth cannula or mouth/ nasal cannula placed in the patient’s mouth or nasal/mouth, permitting tidal breathing. The nostrils were not clipped shut and patients were instructed to breathe normally for 10 seconds. This was repeated three times, following which the average ETCO2 value was recorded. No adverse events were documented during the process of obtaining ETCO2 values. The majority of CTPA scans were conducted within 48 hours of admission and the results were noted. The collected data underwent analysis using logistic regression models to investigate the relationship between PE status, confirmed by a CTPA scan and individual test scores. Predictors were derived from the test scores, while PE status was considered the outcome measure. Additionally, Receiver Operating Curves (ROCs) were utilized to determine the optimal ETCO2 required to differentiate patients with PE from those without, with Area Under the Curve (AUC) serving as the measure of accuracy. A P-value of 0.05 was deemed statistically significant. All analyses were performed using Stata 13 (StataCorp. Stata release 13 statistical software, College Station, TX, USA).
This study conducted a comprehensive assessment of 479 patients, comprising 264 females and 215 males, with ages ranging from 18 to 93 years. Out of all the patients, 136 (25.3%) were diagnosed with Pulmonary Embolism (PE) through Computed Tomography Pulmonary Angiography (CTPA) scanning. The study enrolled 316 patients from the Acute Medical Assessment Unit, with 82 cases of PE and 221 inpatients with 54 cases of PE. The findings of this study indicate the prevalence of PE in the given population and the potential significance of CTPA scanning in diagnosing the condition. It should be noted that none of the patients were enrolled twice in the study. The findings reveal that a considerable proportion of patients diagnosed with Pulmonary Embolism (PE) were also afflicted with multiple co-morbidities (50%) as compared to those in the non-PE group (23%). It was observed that 58% of obese patients and 86% of cancer patients were diagnosed with PE, indicating a significant correlation between these medical conditions and PE.
The present study aimed to evaluate the D-dimer levels in a cohort of 431 patients. The D-dimer levels were measured using monoclonal antibodies and a level of ≥ 275 μg/l was considered positive. Patients with Pulmonary Embolism (PE) had a D-dimer range of 322-8836 μg/l and all patients with PE had a positive D-dimer test. Interestingly, 72% of patients without PE also had a positive D-dimer result. The mean D-dimer in the PE-positive group was 1,855 μg/l (range 289-6,899 μg/l), while the mean D-dimer in the PE-negative group was 609 μg/l. A Receiver Operating Characteristic (ROC) curve was generated and the corresponding sensitivities and specificities are shown in Figures 1A-1C.
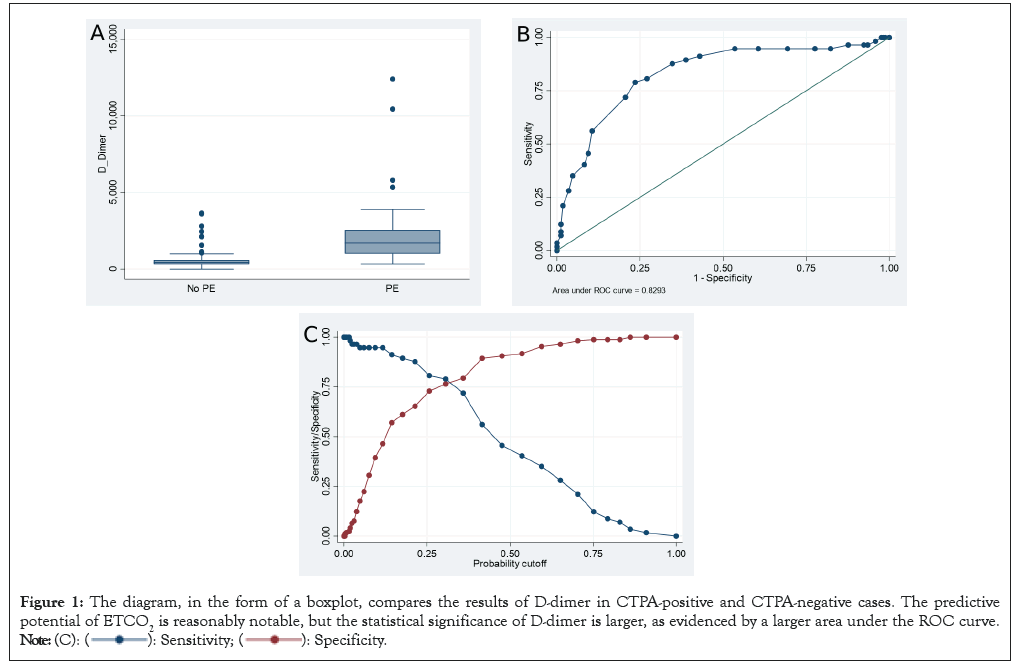
Figure 1: The diagram, in the form of a boxplot, compares the results of D-dimer in CTPA-positive and CTPA-negative cases. The predictive
potential of ETCO2 is reasonably notable, but the statistical significance of D-dimer is larger, as evidenced by a larger area under the ROC curve. 
The findings of this study underscore the importance of D-dimer measurements in the diagnosis of PE. The results demonstrate that a positive D-dimer test alone cannot be used to confirm or exclude PE, as a significant number of patients without PE also had positive D-dimer results. However, the mean D-dimer in the PE-positive group was significantly higher compared to the PE-negative group, indicating the utility of D-dimer measurements as an adjunct diagnostic tool. These findings have important implications for the clinical management of patients with suspected PE, highlighting the need for a comprehensive diagnostic approach that considers multiple clinical and laboratory parameters.
The Receiver Operating Characteristic (ROC) curve is an effective tool in demonstrating the diagnostic ability of ETCO2 in differentiating between patients with Pulmonary Embolism (PE) and those without. Figures 2 and 3 exhibit the corresponding sensitivities and specificities, with an Area Under the Curve (AUC) of 0.84. In patients who tested negative for PE, the average ETCO2 was observed to be 32.53 mmHg. Notably, this point had a sensitivity of 100% and specificity of 68%, with a Negative Predictive Value (NPV) of 100% and a Positive Predictive Value (PPV) of 66%. This data reveals that 32.53 mmHg was the lowest point at which the sensitivity and NPV achieved 100% while maintaining high specificity.
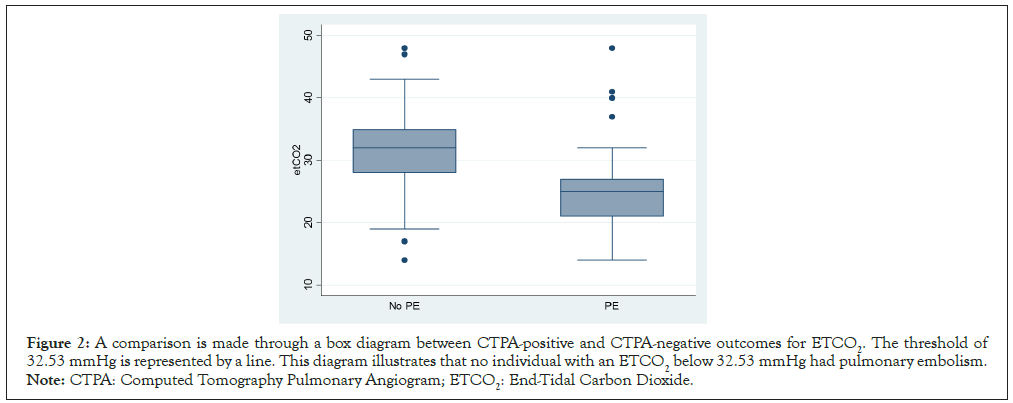
Figure 2: A comparison is made through a box diagram between CTPA-positive and CTPA-negative outcomes for ETCO2. The threshold of 32.53 mmHg is represented by a line. This diagram illustrates that no individual with an ETCO2 below 32.53 mmHg had pulmonary embolism. Note: CTPA: Computed Tomography Pulmonary Angiogram; ETCO2: End-Tidal Carbon Dioxide.
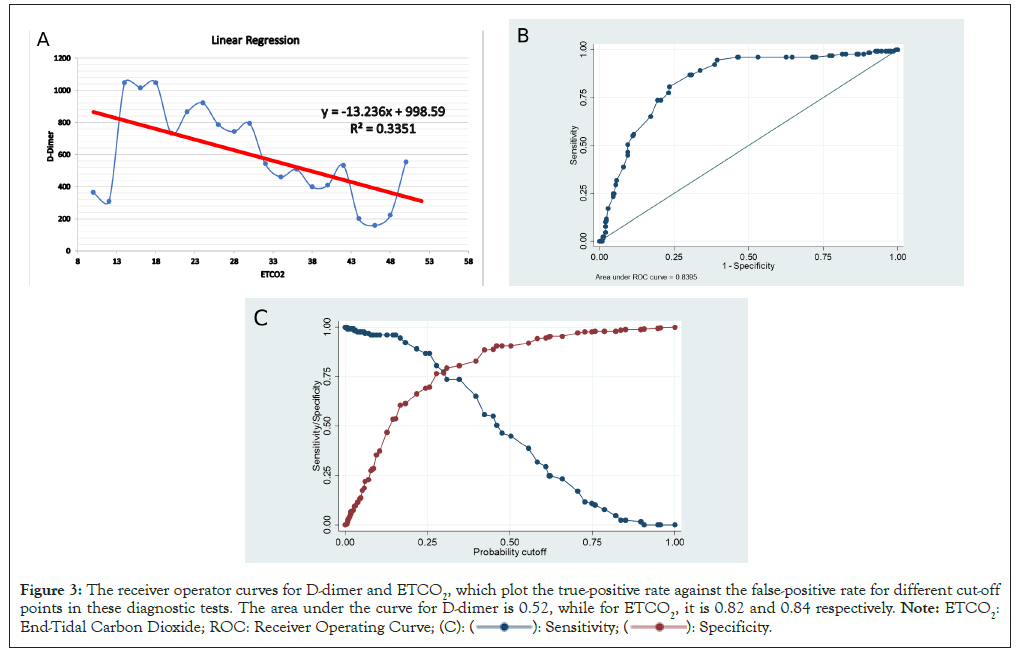
Figure 3: The receiver operator curves for D-dimer and ETCO2, which plot the true-positive rate against the false-positive rate for different cut-off
points in these diagnostic tests. The area under the curve for D-dimer is 0.52, while for ETCO2, it is 0.82 and 0.84 respectively. Note: ETCO2:
End-Tidal Carbon Dioxide; ROC: Receiver Operating Curve; 
Among 136 patients with PE, 14 patients were observed to have ETCO2 values exceeding 30 mmHg, indicating a false negative occurrence. Conversely, in 381 patients without PE, 6 patients exhibited ETCO2 values less than 20 mmHg, resulting in a false positive outcome. Notably, the ETCO2 value of 34.2 corresponded to a 10% cut-off, with a sensitivity of 96% and a specificity of 37% (Table 1).
| ETCO2 mmHg | Predictive N=4679 | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|
| 24.1 | >=50% | 44.96% | 90.57% | 63.74% | 81.70% |
| 29.1 | >=25% | 86.82% | 69.71% | 51.38% | 93.49% |
| 34.2 | >=10% | 96.12% | 37.43% | 36.15% | 96.32% |
Table 1: The table with classified ETCO2 presents a variety of cut-off thresholds.
Machine learning based modelling
Based on the statistical significance and the strong correlation between various variables under consideration, we have embarked upon a crucial inquiry to determine the feasibility of developing a predictive machine model. Specifically, we seek to ascertain whether it is possible to predict the approximate values of D-dimer and CTPA based on the value of ETCO2. Our objective is to establish a reliable and accurate machine-learning algorithm that can effectively predict these outcomes.
In order to address our research problem, we conducted an investigation into the application of machine learning-based regression modelling. After careful consideration, we determined that decision tree-based regression modelling would be most advantageous for this problem. Our decision was since the available data is diverse and limited in scope and the desired outcomes are also confined to a specific domain. We obtained the raw data, which is graphically illustrated in Figure 4 and used it to develop the necessary learning for our model.
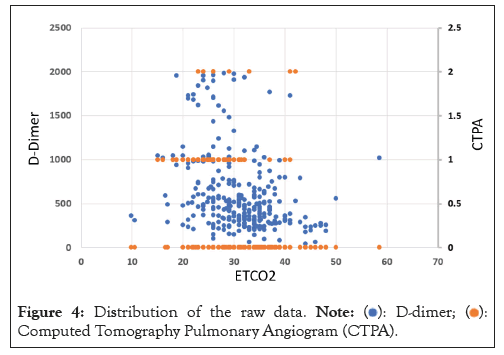
Figure 4: Distribution of the raw data.  Computed Tomography Pulmonary Angiogram (CTPA).
Computed Tomography Pulmonary Angiogram (CTPA).
The decision tree based machine learning method is widely recognized for its ability to build models with noisy and largely missing data. This approach involves constructing a regression tree through a process called recursive partitioning, which partitions the data into branches and continues the process throughout the dataset. The resulting model is highly interpretable and can be used to make accurate predictions, even when the data is complex and difficult to work with. This method is particularly useful in business and academic settings where data is often incomplete or contains a significant amount of noise. By leveraging decision tree-based machine learning, organisations can gain valuable insights from their data and make informed decisions that drive success. In the present process, the algorithm guarantees that the sum of squared deviations of the data points from the mean is minimised for a given branch in the dataset. This methodology is consistently followed throughout to ensure an excellent fit to the available data, while also possessing the ability to predict new values via regression-based interpolation. The algorithm’s precision in minimising the square deviations of the data points from the mean results in an optimal fit that can be utilized in the prediction of new values. This process is particularly useful for businesses and academic settings, where accurate data analysis is crucial for decision-making.
The plots in Figure 5 are the outcome of a regression model based on a decision tree. The raw data was fed to the algorithm, which utilised its interpolation capacity to predict new values for the variables. Specifically, the predicted values of CTPA and D-dimer were calculated by feeding the ETCO2 values ranging from 10 to 50 into the model. Based on the analysis presented in Figure 5, the decision regression model can be used to estimate both the D-dimer and CTPA for values of ETCO2 ranging from 20 to 50.
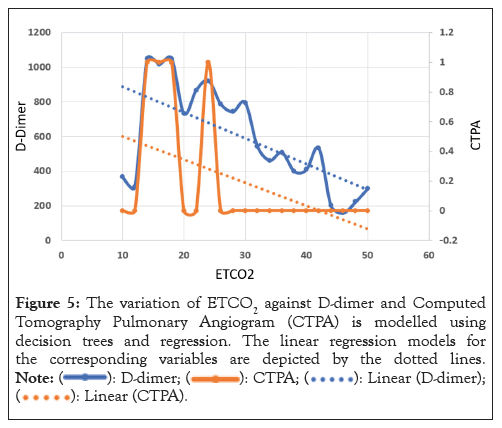
Figure 5: The variation of ETCO2 against D-dimer and Computed
Tomography Pulmonary Angiogram (CTPA) is modelled using
decision trees and regression. The linear regression models for
the corresponding variables are depicted by the dotted lines. 
PE affects 0.5-1% per 1000 people and is the leading cause of death among hospital inpatients. Early diagnosis is crucial to prevent such events. Doctors may face difficult situations when the patient’s vital signs deteriorate without any clear cause and diagnostic tests may not be readily available due to the patient’s instability or local conditions [14].
Metin Ozdemir, et al., included 100 consecutive subjects suspected to have PE and a positive D-dimer test to study clinical probability of PE and ETCO2 levels. ETCO2>34 mmHg was found to be the best cut-off point for diagnosing PE. In their experience, PE was ultimately eliminated or diagnosed by spiral Computed Tomography (CT). Diagnostic performances of tests were as follows: ETCO2 and D-dimer had a sensitivity of 100% and a Negative Predictive Value (NPV) of 100% at the cut-off levels of 34 mmHg and 500 ng/mL, respectively; Wells score had a sensitivity of 80% and NPV of 69.7% at a score of 4. Therefore, they concluded that ETCO2 alone cannot reliably exclude PE. Combining it with clinical probability, however, reliably and correctly eliminates or diagnoses PE and prevents further testing from being done [15].
However, data gathered from our previous work conducted in 2012 had elucidated that ETCO2 is a quick, safe, reliable and non-invasive bedside test in excluding PEs. This study was conducted as a follow-up study in which the main focus remained on validating the use of ETCO2 level routinely in patients suspected to PE as a practical, cheap tool with no adverse side effects at the accident and emergency departments, first responders and hospitalised patients. The data gathered from this prospective study showed that out of 38 patients who were diagnosed with a PE, none of the patients attained an ETCO2 value of ≥ 4.3 kPa (32.3 mmHg). In the current assessment, included a large number of patients (n=479) who were suspected of having a PE and with this large cohort, the sensitivity of using ETCO2 value as a diagnostic marker for PE presented with (an ETCO2 of 32.7 Sn=93%; Sp=48%) 93% sensitivity (Figure 2). Additionally, this study also assessed patients who had existing underlying diseases such as cancers, immobility, COPD, diabetes, lupus and other autoimmune diseases as well as evaluating those without any background history. Previously, it has been proposed that valuable tools for excluding PE include the assessment of alveolar dead space ventilation and the expired tracer gas CO2 as surrogates for pulmonary vascular obstruction [13]. The threecompartment model of the lung is the basis of this fundamental pathophysiological principle. The three-compartment model of the lung consists of an ideal compartment that is ventilated and perfused, a shunted compartment that is perfused but not ventilated and the final compartment that is ventilated but not perfused (dead space). The third compartment, otherwise known as the dead space, comprises the anatomic dead space as the ventilated airway space and alveolar dead space as the volume of unperfused alveoli. By measuring the CO2 arterial tension to end-tidal CO2 gradient as a percentage of the ventilated but not perfused lung, one can determine the approximate size of the alveolar dead space. Moreover, the size of the alveolar dead space has been noted to increase in thromboembolic obstruction [13]. Moreover, previous research has shown the ability to rule out a PE by combining alveolar dead space fraction calculations and plasma D-dimer assays.
Our previous study elucidated that a positive D-dimer ( ≥ 275 µg/l) and an ETCO2 value of less than 32.25 mmHg obtained a sensitivity value of 85%, the specificity of 64% and PPV and NPV of 60% and 87%, respectively, when used in combination to determine a positive PE result. Within the cohort, three patients testing negative for a PE attained an ETCO2 reading of less than 17.25 mmHg as well as also having a positive D-dimer test. The results obtained highlighted inappropriateness of using a D-dimer testing for DVT exclusion and PE, which can, in turn, lead to misdiagnosis, overuse of imaging study and unnecessary cost of D-dimer testing [16,17]. Moreover, out of 136 patients who were diagnosed as PE positive, 14 patients had an ETCO2 value of more than 30 mmHg, making the percentage of attaining a false negative 10.3%. Furthermore, out of 381 patients who received no PE result, six patients had an ETCO2 value of less than 20 mmHg, thus making the percentage of attaining a false positive 1.6%. This data is essential as attaining a false positive can ultimately lead to unnecessary treatment and use of valuable equipment, whereas obtaining a false negative can lead to a false diagnosis, which in itself is very serious.
In conclusion, there is a huge value in measuring ETCO2 in patients presenting pulmonary embolism signs and symptoms. In particular, by applying the data from Figures 4 and 5, the values ETCO2 between 20 and 50, both the D-dimer and CTPA can be approximated using the decision regression model.
As a result of this for future studies, it will be interesting to look into developing an accurate as well as robust machine learning model to improve the diagnosis of PE at the critical stages. In order to generate an effective machine learning algorithm, we will need to obtain a sufficient and labelled dataset along with we must also identify an efficient computational framework such as deep learning. This should eventually provide an effective and efficient system for automated diagnosis of PE.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Jacob B, Shahzad F, Ugail H, Walker J, Scally A, Najafzadeh M (2024) Using End-Tidal CO2 Measurement as a Tool to Diagnose Pulmonary Embolism. Trans Med. 14:317.
Received: 26-Apr-2024, Manuscript No. TMCR-24-30962; Editor assigned: 29-Apr-2024, Pre QC No. TMCR-24-30962 (PQ); Reviewed: 13-May-2024, QC No. TMCR-24-30962; Revised: 20-May-2024, Manuscript No. TMCR-24-30962 (R); Published: 27-May-2024 , DOI: 10.35248/2161-1025.24.14.317
Copyright: © 2024 Jacob B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.