Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2023)Volume 11, Issue 6
This study was conducted to investigate the possibility of using existing vectors as an heterologous system to silence genes in other plant species. In this study I measured the effectiveness of pre-existing Virus Induced Gene Silencing (VIGS) vector as a tool for gene silencing in Ipomoea purpurea, Ocimum basilicum and Anethum graveolens. The results showed that TRV-based VIGS vector with heterologous Phytoene Desaturase (PDS) sequences from Nicotiana bentamiana silenced the endogenous PDS gene in Ipomoea purpurea but not in Ocimum basilicum and Anethum graveolens.
Virus induced gene silencing; Heterologous system; Ipomoea purpurea; TRV based vector; Phytoene desaturase
It is very difficult for some plants to introduce many traits through classical breeding and also performing stable genetic transformation in these plant species. Therefore, development of a rapid and powerful tool for plant functional genomics and validation a gene for loss of function could be important. Metabolic engineering by using methods such as gene silencing efforts to elucidate secondary metabolites pathways and to generate a temporal and spatial control over gene expression in medicinal plants [1].
There are approximately 58 genera and 2000 species in the convolvulaceae family that some of these species are commercially important. Ipomoea is one of the most consequential genera which belongs to this family and contains 600 species-700 species. One of these species like Ipomoea nil became the subject of intense research concerning their potential to study the genetic basis of flower colouration and morphology as a model plant. Molecular studies showed that the convolvulaceae are now founded as a sister group of solanaceae. It can be a good subject to use TRV based heterologus VIGS system in this family. Virus-Induced Gene Silencing (VIGS), as RNA interference based technology has become a widely used forward and reverse genetics tool for gene function and functional genomics in various higher plants. Tobacco Rattle Virus (TRV) used as viral vector to silence genes in a broad range of host plants including several solanaceae plants [2].
Existence of a large number of publicly available gene sequences as a result of expression analyses and gene discovery creates the need to employ methods to study gene function. To date various VIGS vectors have been developed the limitation of host range of viral vectors, even with the TRV virus, which is restricted to the solanaceae family, has resulted in the necessity of using other potential of this method, such as the heterologous system. It has been reported that the use of heterologous gene sequences, even from distantly relative species with minimum nucleotide sequence homology can lead to trigger gene silencing in the model plants [3]. Some of these studies were dedicated to silence genes in species with remote evolutionary relations such as a fragment of PDS gene in Lilium longiflorum and a DEAD box helicase gene from Dunaliella salina to silence its ortholog in N. benthamiana. Also using the Arachis hypogaea LEA4 gene sequence (a drought induced peanut gene) can cause silencing of endogenous gene in tomato [4]. Very few studies have been made about using heterologous VIGS system to induce silencing in non-model plants. First report of gene silencing associated with sequence homologies has been reported in members of solanaceae family including tomato, tobacco (Nicotiana tabacum) and Petunia hybrid using the N. benthamiana PDS gene sequence. In another study, heterologous gene sequence tobacco PDS (NbPDS) and tomato RBCS (SlRBCS) were used to silence its orthologs for VIGS in various plant species belonging to the solanaceae family (Nicotiana benthamiana L., Nicotiana clevelandii, Nicotiana plumbagenifolia L., Nicotiana glutinosa L., Solanum lycopersicum L. (tomato; varieties arkavikas and microtom), Solanum pimpinellifolium L., Solanum peruvianum L., Solanum cheesmaniae. Because virus induced gene silencing is a dependent method to sequence homology the amount of sequence similarity or identity of heterologous gene with the endogenous gene had a lot of influence on the efficiency of gene silencing [5]. In general, the lower limit of nucleotide identity for optimal results of gene silencing has been reported 85%. In order to development of the domain of using viral vector to different plant species, in this study, we developed the first heterologous VIGS system in Ipomoea purpurea using TRV as a vector. Our results illustrated that heterologous VIGS system are able to analysis of which help to cost effectively gene function of using existing vectors as an heterologous system to silence PDS gene in these plants [6].
Plant material and growth conditionsFor VIGS treatment, Nicotiana benthamiana L., Ipomoea purpurea, Ocimum basilicum and Anethum graveolens were grown in plastic pots containing peat in growth rooms at 22℃-25℃ with 60% relative humidity and a 16 h photoperiod [7].
Construction of TRV plasmids
The VIGS experiment was performed as previously described by Senthil-Kumar and Mysore. The pTRV2 vectors were used to express PDS from Nicotiana benthamiana. Oligonucleotide primer used for cloning were forward 5- TTATGAATTCATGCAGAACCTGTTTGG-3 and reverse 5 TTATGGATCCGTTAAGTGCCTTTGAC-3 that designed to amplify a fragment of 344 bp of the PDS gene. PCR amplicons were cloned into the multiple cloning sites of pTRV2-MCS using the restriction sites. Agrobacterium tumefaciens carrying PDS was transformed along with empty pTRV2 vector and pTRV1 vector. The A. tumefaciens strain GV3101 harbouring pTRV1 or pTRV2 and its derivatives were grown at 28℃ in Luria-Bertani (LB) medium containing appropriate antibiotics [8].
The harvested cells was inoculated into 10 ml induction medium (10 mM MES, pH 5.5, and 200 μ Macetosyringone) followed by incubating them at room temperature in a shaker (50 r.p.m.) for 3 h. The harvested cells re-suspended in 5 mL infiltration buffer (5 mM MES, pH 5.5) to a final absorbance (Optical Density (OD) at 600 nm) of 1.0. For leaf infiltration, each A. tumefaciens strain containing pTRV1 or pTRV2 and its derivatives were mixed at ratio of 1-1. Plants in one leaf stage were infiltrated using 1 ml needless syringe. The plants were maintained at 24℃ in a growth chamber [9].
Pigments measurement
Carotenoid, chlorophyll a and b content in leaf tissue was measured using Arnon’s method. For all plants in the experiments, TRVWT inoculated green leaves and TRVNbPDSinoculated white dot leaves were analysed. Finally, pigments contents were calculated as described previously [10].
Gene expression analyses
RNA was isolated from leaf tissues of Nicotiana benthamiana L. and Ipomoea purpurea using PBIOZOL reagent (Bioer technology, China) according to supplier’s recommendations and first strand cDNA was synthesized using oligo(dT)15 primers. The cDNA from each species was used to amplify the PDS gene sequence by Polymerase Chain Reaction (PCR) using gene-specific primers. Quantitative RT PCR was performed in light cycler 96 (Roche Co.) using the specific primers for the PDS gene forward 5-AGGCGGCGTTATTATCATC-3 and reverse 5´-CAATCTCCATCATCATCTTTCC-3 and the EVA green qPCR Mix Plus5X HOT FIRepo [11]. The rbcL reference gene primers were used as an internal control to ensure cDNA synthesis. Relative gene expression level was measured using the ΔΔCT method and were normalised against rbcL.
Statistical analysis
Student’s t-test was used for statistical analysis using SAS 9.3 (SAS institute Inc., Cary, NC, USA) and IBM SPSS statistics 24. Statistical significance was set at P<0.05 [12].
We studied the competency of using heterologous VIGS system to induce silencing by monitoring leaf photobleaching caused by Phytoene Desaturase (PDS) silencing in Ipomoea purpurea, Anethum graveolens and Anethum graveolens. For this purpose, a 344 bp of the NbPDS gene was cloned into the pTRV2. Both constructs containing the pTRV1 and pTRV2 mixed and then were introduced into plant cells via Agrobacterium inoculation. At 10 days-12 days post infiltration, along leaf veins in newly emerged leaves exhibited partially photobleaching phenotype in all pTRV2-PDS-transformed plants in Ipomoea purpurea and Nicotiana benthamiana. Whereas control treatments did not show any outward symptoms associated with PDS gene silencing (Figure 1) [13]. Also, in the other two species no signs were visible. One of the advantages of this method is the absence of phenotypic differences between the uninfected plants and the plants injected with the empty vector [14].
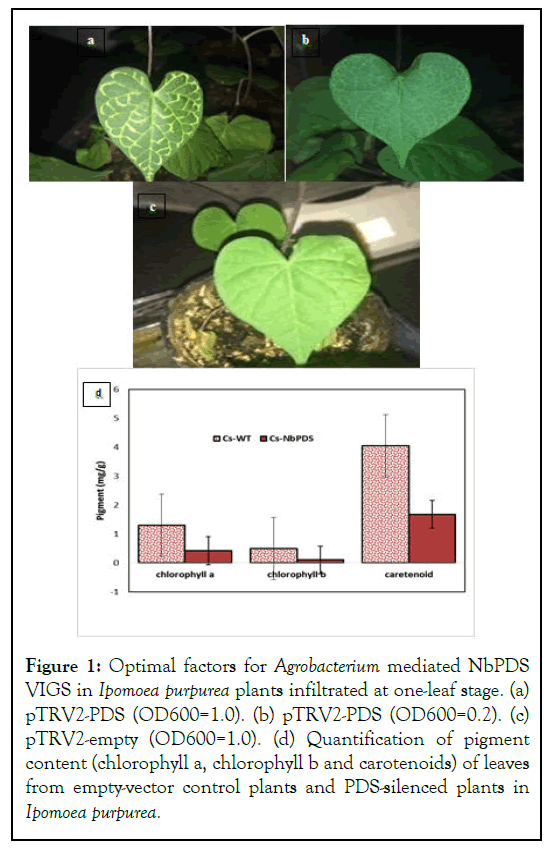
Figure 1: Optimal factors for Agrobacterium mediated NbPDS VIGS in Ipomoea purpurea plants infiltrated at one-leaf stage. (a) pTRV2-PDS (OD600=1.0). (b) pTRV2-PDS (OD600=0.2). (c) pTRV2-empty (OD600=1.0). (d) Quantification of pigment content (chlorophyll a, chlorophyll b and carotenoids) of leaves from empty-vector control plants and PDS-silenced plants in Ipomoea purpurea.
Optimal conditions for TRV VIGS
In our experiment to evaluate the effect of conditions for heterologous TRV based VIGS in Ipomoea purpurea, Ocimum basilicum and Anethum graveolens, we investigated whether seedling growth stage and bacterial inoculum concentration (OD600=0.2 and OD600=1.0) affected the quality of silencing Phytoene desaturase (NbPDS) gene as a marker in Ipomoea purpurea. In Ipomoea purpurea, the result of growth stage on effectiveness of silencing on treated plants showed that no differences between plants in silencing were observed in two stages of development (cotyledon and one leaf stage) [15]. 100% of plants treated in both growth stage displayed photobleaching indicative of PDS silencing. Also, the plants infiltrated to bacterial density OD600=1 exhibited the greatest effect on VIGS as compared OD600=0.2. All infiltrated plants displayed photobleaching symptoms. Sweet basil and common dill plants injected with silencing constructs at cotyledon and 2 leaf stage did not show any visible signs of PDS gene silencing, while in the model N. benthamiana plant gene silencing signs were visible in the second week after infiltration.
The chlorophyll contents were assayed to determine the extent of photobleaching in leaves. Chlorophyll a content was lower in photobleached TRVNbPDS inoculated I. purporea than in green control plants. Leaves with a visible phenotype showed a decrease of chlorophyll a, b and carotenoids by 32%, 20% and 41.48% in I. purporea.
In Ipomoea plants infilterated by heterologus PDS silencing construct, no completely white leaves were observed but small white spot in leaves indicated down regulation of transcript level of the PDS gene [16].
Ipomoea seedlings showed photobleaching on upper leaves of all treated plants limited to main veins of newly formed leaves.
It seems that phylogenetic relationships could play a role in the success of the heterologus silencing method. Other studies on heterologous VIGS system showed that different degrees phenotype of leaf bleaching in N. benthamiana was triggered by PDS fragment from other species even distant relative species [17]. Benedito, et al., showed that VIGS may work in heterologus dicot system in N. benthamiana using a monocot gene fragment from Lilium longiflorum. The lily PDS fragment showed 70% identity to PDS from N. benthamiana. Our results indicated that a gene fragment of phytoene desaturase derived from N. benthamiana is able to elicit some degree of silencing in I. purpurea.
PDS gene expression
Real time PCR analysis was conducted to assess endogenous transcript levels of PDS in Ipomoea purpurea, the results showed that endogenous transcript levels of phytoene desaturase gene are reduced 2.5 fold in photobleached plants compared with the control plants. Relative IpPDS levels were normalized against rbcL. Nicotiana benthamiana photobleached showed approximately 4.1-fold reduction in levels compared with control. In a study on silencing of the PDS gene in N. benthamiana using heterologous PDS gene sequences from woody plant species (PDS fragments from Populus tomentosa Carr., Camellia oleifera and Vernicia fordii) only 50% reduction was observed in plants infected with TRV2- PtoPDS compared with the control [18]. In the silencing of the VfPDS gene by heterologous gene fragments from P. Tomentosa Carr the target mRNAs were reduced to approximate 75% in the targeted gene silenced plants (Figure 2).
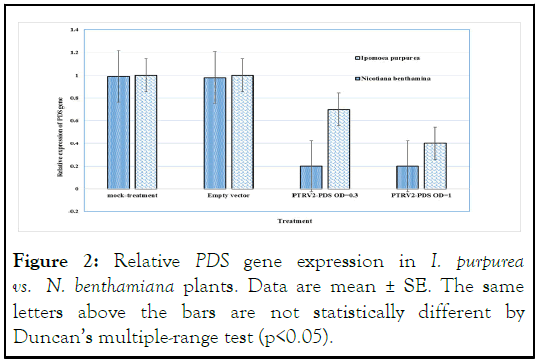
Figure 2: Relative PDS gene expression in I. purpurea vs. N. benthamiana plants. Data are mean ± SE. The same letters above the bars are not statistically different by Duncan’s multiple-range test (p<0.05).
The large volume of information generated by high throughput techniques for gene discovery and expression analysis have increased the demand for efficient procedures to unravel gene functions, in order to make them useful for fundamental and applied purposes, the VIGS system, can potentially be provided this opportunity as an important tool in reverse genetic analysis, to ease and speed up analysis of gene function without the requirement of time-consuming transformation and tissue culture procedures, however it has been recently demonstrated that commercially available constructs from closely or some time distant related species can be used as a heterologous virus induced silencing system to assess gene function in new species especially for non-model plant species where little sequence information is available [19].
In comparison to stable transformation, Virus-Induced Gene Silencing (VIGS) introduced as a rapid and powerful tool for plant functional genomics. More importantly, this method can improve its speed and efficiency by using heterologous gene sequences to silence orthologs in plants that do not have extensive gene sequence information. In the present study, we developed the first TRV-VIGS system in Ipomoea purpurea. Although in some reports have demonstrated that 100%similarity between heterologous sequences and the target gene for efficient silenceing by VIGS is essential, there is no consensus on this issue. However, in several studies, at least the similarity of sequence is 85% [20].
In our experiment, PDS sequence from Ipomoea purpurea (Ipomoea sp. Kenyan PDS mRNA for phytoene desaturase, complete cds had identities of 85% and 80% with the N. benthamiana host gene. Previous studies have shown that environmental conditions including temperature, optical density of bacterial inoculum and growth stage have affected on the silencing efficiency [21].
The incidence of silencing symptoms in the Ipomoea purpurea leaves confirms that silencing process in this plant depends on the early growth phase. It has been reported that the improvement of plant resistance to pathogens during maturation process may reduce the VIGS efficiency. Optimizing PDS virus-induced gene silencing efficiency using TRV with Arabidopsis thaliana showed that the best result obtained at a 2- leaf to 3-leaf stage in compared to higher growth steps. Previous studies have shown that young seedlings of plants may have a particularly competent stage for Agrobacterium infiltration in VIGS. Also, some reports have examined the influence of agroinoculation concentration in gene silencing efficiency in some plant species. According to these studies, the culture concentration has a significant effect on silencing PDS gene, so that the best result of silencing is obtained in the OD600=1.5 or higher bacterial concentrations in Gossypium barbadense.
Silencing of endogenous PDS and cloroplastos alterados 1 gene (CLA1) in tung tree (V. fordii) with heterologous PtoPDS and PtoCLA1 gene sequences from P. tomentosa Carr. As well as CoPDS sequence from C. oleifera also was reported in the Agrobacterium inoculum with a final OD600 of 1.0. Our results suggested that inoculation of early growth seedling and an OD value of 1 for the Agrobacterium culture could promote the photobleaching phenotype triggered by NbPDS gene silencing [22-25].
Using heterologus system for virus induced gene silencing suggested the potential of this system for speeding up and simplified functional characterization of the many genes for new species for which function has yet to be discovered or confirmed.
For optimal results, leading to specific, effective and reliable gene silencing in a heterologous system, the establishment of the level of sequence homology that is required is pivotal information. It is generally assumed that 85% nucleotide identity would be the lowest limit for triggering the silencing mechanism; however, there is no experimental evidence. Possibly in the near future this field will be open for speedy and simplified functional characterization of the many genes from new species, for which function has yet to be discovered or confirmed.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Borna T (2023) Using Heterologous Virus-Induced Gene Silencing in Ipomoea purpurea as a Sister Family of Solanceae. J Plant Biochem Physiol. 11:271.
Received: 18-Mar-2023, Manuscript No. JPBP-23-22272; Editor assigned: 21-Mar-2023, Pre QC No. JPBP-23-22272 (PQ); Reviewed: 04-Apr-2023, QC No. JPBP-23-22272; Revised: 25-May-2023, Manuscript No. JPBP-23-22272 (R); Published: 01-Jun-2023 , DOI: 10.35248/2329-9029.23.11.271
Copyright: © 2023 Borna T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.