
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2024)Volume 14, Issue 5
Background: Sexually Transmitted Infections (STIs) and Human Immunodeficiency Virus (HIV) in the U.S. Military population impair servicemembers’ ability to perform their duties, negatively impacting a unit’s operational capability in support of national defense. As such, the increasing incidence of STIs in the U.S Military is of significant concern. Sexual behaviors are a key driver of STI incidence, and the U.S. Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend evidence-based behavioral interventions targeting the reduction of health risk behaviors as part of a comprehensive approach to STI/HIV prevention. However, there is no behavioral intervention with demonstrated efficacy in reducing high-risk sexual behaviors and the incidence of STI/HIV in the military population.
Methods: A prior pilot study of an evidence-based behavioral intervention adapted for the military population, the Knocking-out Infections through Safer-sex and Screening (KISS) intervention, demonstrated initial feasibility and acceptability in active-duty U.S. Army personnel and their medical beneficiaries. Based on pilot findings, we designed and implemented a multi-site randomized controlled trial to reduce high-risk sexual behaviors and STI/ HIV incidence in a behaviorally vulnerable population of U.S. Army personnel and their medical beneficiaries. Here we describe the design and implementation of the randomized controlled trial.
Conclusion: The results of this study will inform best-practices and policy regarding STI/HIV prevention in the U.S. Military population to potentially provide a much-needed evidence-based intervention to temper the increasing incidence of STI/HIV among the U.S. Military population.
Clinical trials registration number: NCT04547413
Sexually transmitted infections; Human immunodeficiency virus; Behavioral intervention; Military
Human Immunodeficiency Virus (HIV) and other Sexually Transmitted Infections (STIs) impair servicemembers’ ability to perform their duties, which has a profound negative impact on a unit’s operational capability in support of national defense. As such, the increasing incidence of STIs among servicemembers is of significant concern. The annual incidence rate of chlamydia among active duty servicemembers increased 67% to 240/10,000 person- years from 2013 to 2019 [1]. Chlamydia’s annual incidence rate among active duty servicemembers then decreased in 2020-2021 to 180/10,000 person-years, mirroring the trend in the general U.S. population [1]. Given that chlamydia is often asymptomatic, the recent decrease may result from COVID-19 pandemic-related declines in testing and reporting of cases rather than an actual decrease in new infections [1,2]. The annual incidence rate of gonorrhea among active duty service members increased 41.8% to 34/10,000 person-years, while syphilis increased 52.5% to 6/10,000 person-years from 2013 to 2021 [1].
HIV incidence has remained relatively stable recently among active duty servicemembers (16 incident HIV diagnoses per 100,000 in 2021) [3]. However, the impact of duty limitations and implications of a non-curable chronic infection requiring lifelong therapy on mission readiness, the individual servicemember’s career, and the associated potential adverse health outcomes, even in those with well-managed infection, impart particular concern.
The 2018 Department of Defense Health Related Behaviors Survey reported a high prevalence of STI/HIV risk behavior in the U.S. Military population [4]. Among active duty servicemember respondents, nearly 20% reported multiple sex partners, nearly 35% reported sex with a new partner without using a condom, and just over 75% reported not using a condom the most recent time they had vaginal sex within the past year [4]. Additionally, 22% of respondents were considered at high risk of HIV, defined as having multiple sexual partners, an STI in the past year, or a Male reporting Sex with other Men (MSM) [4].
Targeting the reduction of high-risk sexual behaviors through behavioral interventions effectively reduces the incidence of HIV and other STIs [5]. The U.S. Centers for Disease Control and Prevention (CDC) and the U.S Preventive Services Task Force recommend behavioral interventions targeting reduction in high- risk sexual behaviors as part of a comprehensive approach to STI/HIV prevention [5,6]. However, nearly all of the behavioral interventions with demonstrated efficacy in reducing STI/HIV incidence have been evaluated in non-military populations [7]. Of the few behavioral interventions that have been studied in a military population, only three included STI incidence as an outcome measure [8-10]. Only 1 of those studies, which was limited to female U.S. Marine recruits at a single base, demonstrated efficacy in reducing STI incidence [9]. Despite the robust evidence for the efficacy of behavioral interventions in reducing STI/HIV incidence in non-military populations, it cannot be assumed that similar interventions will be effective in the military population given its unique diversity, mobility, and culture. As such, there remains a need to develop evidence-based behavioral interventions to reduce the burden of HIV and other STIs in the U.S. Military population.
To begin addressing this need, a feasibility and acceptability pilot study of an evidence-based STI/HIV behavioral intervention program adapted for the military population was previously conducted at Joint Base Lewis-McChord (JBLM), WA among U.S. Army personnel and their medical beneficiaries [11]. This pilot intervention, Knocking-out Infections through Safer-sex and Screening (KISS) was adapted for use in the military population from HORIZONS, a U.S. CDC-defined evidence-based behavioral intervention [7]. HORIZONS is based on Social Cognitive Theory and Theory of Gender and Power and includes STI/HIV prevention knowledge, condom use, and regular STI/HIV screening content [12]. HORIZONS has been shown effective in increasing condom use and reducing STI incidence in a population of young African American women [12]. For the KISS intervention, HORIZONS was modified for relevance across the diversity of gender, race, and ethnicity present in the U.S. Military population using the ADAPT-ITT model, a framework for adapting evidence-based STI/HIV behavioral interventions to new target populations with behavioral vulnerability [13]. Vignettes were developed to represent different gender, racial and ethnic identities including multi-racial/ethnic individuals, and the content was modified to include greater focus on role of substance use and intimate partner violence in STI/ HIV transmission given evidence suggesting high prevalence of substance use and coercive sex in military populations [14,15].
The KISS pilot study demonstrated feasibility in implementation and acceptability to the target military/military medical beneficiary population, with 83.5% of participants attending the single intervention session and 69.7% returning for the final 3-month follow-up assessment [11]. Nearly all participants reported feeling comfortable with their group (97%) and asking questions in the group session (95%), that they were more likely to practice safe sex after the intervention (92%), and that they would recommend the intervention to friends (92%) [11]. Additionally, in pre/post- intervention testing on STI/HIV prevention knowledge, scores significantly increased immediately after the intervention (34.8% increase in correct responses compared to pre-intervention) and was maintained at 3 months (20.6% increase in correct responses compared to pre-intervention) [11]. While the study was not designed to assess changes in sexual behavior or STI incidence, no participants were diagnosed with an STI at the final study visit (compared to 18.9% diagnosed with chlamydia or gonorrhea at enrollment), and reported condom use at last sexual encounter increased by 10.3% [11]. Based on the results of this pilot study, the KISS behavioral intervention program has significant potential to fill the void of a much-needed evidence-based intervention to reduce the incidence of STI/HIV in the U.S. Military population.
Study aim
The aim of this study is to further assess the efficacy and acceptability of the KISS adapted behavioral intervention program in changing high-risk sexual behaviors and decreasing rates of STI/HIV infections within a high-risk population of U.S. Army personnel and their medical beneficiaries.
Study design
This study is a multi-site randomized controlled trial involving U.S. Army personnel and their adult medical beneficiaries with behavioral vulnerability to HIV and other STIs as determined by diagnosis of an STI or a clinical indication for STI/HIV screening within the previous 180 days. Participants who meet eligibility criteria and provide informed consent are randomized to either the intervention or control arm. The intervention arm receives the KISS adapted behavioral intervention program in addition to standard STI/HIV prevention counseling routinely provided by military medical treatment facilities. The control arm receives only standard STI/HIV prevention counseling. Both arms receive follow- up contact via SMS/text messaging monthly for 10 months and have follow-up study visits at 6 months and 12 months. During the follow-up visits participants complete electronic questionnaires to assess sexual behaviors and STI/HIV knowledge, and undergo testing for Neisseria Gonorrhoeae (NG), Chlamydia Trachomatis (CT), syphilis, and HIV.
Study setting
Study activities take place at the Madigan Army Medical Center (MAMC) Preventive Medicine clinic at JBLM, WA and the Womack Army Medical Center (WAMC) Epidemiological and Disease Control clinic at Fort Liberty, NC. These clinics are located at military installations with large active-duty U.S. Army and military medical beneficiary populations that are expected to be representative of these populations at-large.
Eligibility
Eligible participants are: (1) active-duty U.S. Army personnel or military medical beneficiaries; (2) age 18–40 years; (3) eligible to receive care at a military healthcare facility for at least 12 months from enrollment; (4) not living with HIV; (5) not scheduled to change duty station or deploy within 3 months from enrollment; (6) not pregnant; (7) not trying to become pregnant or impregnate a partner; (8) at “high-risk” for STI/HIV based on a STI diagnosis or STI/HIV screening in the last 180 days (NG, CT, syphilis and/or HIV; excluding biennial routine HIV testing for Force Protection purposes); (9) reporting vaginal, anal, and/or oral sex in the last 30 days. Military medical beneficiaries are included in this study as this population has similar age, diversity, mobility, and culture to the military population with expected similar incidence of STI/HIV, prevalence of high-risk sex behaviors, and impact of a behavioral intervention adapted to the military population [16].
Recruitment
Participants are recruited actively from persons seeking care at the MAMC and WAMC Preventive Medicine clinics, and passively using flyers strategically placed in public locations at JBLM and Fort Liberty and distributed electronically through email and social media. Participants are also recruited via referral through enrolled participants who are provided with packets containing information about the study and a referral coupon.
Sample size
Based on an expected meaningful proportional difference in incident STI rates of 0.15 between the treatment and control group and specifying an alpha of 0.05 and 80% power, sample size calculations using chi-square testing resulted in a minimum of 350 participants needed in each of the control and intervention arms. Although the plan is to enroll roughly half of the study participants from each site, there are no restrictions on the number of subjects that can be enrolled at each site. To maintain an adequate sample size and study power, participants who withdraw or are lost to follow up by 6 months may be replaced by new participants on a one-to-one basis. Participants that are recruited as replacements may complete less than 12 months but a minimum of 6 months of follow-up at study closure.
Study procedures
A schedule of enrollment, interventions, and assessments is provided in Table 1, and a trial-flow diagram in Figure 1. Study personnel at the MAMC and WAMC clinics review patient appointments in the Electronic Medical Record (EMR) system and/or hard copy charts for walk-in patients. Active-duty U.S. Army personnel and military medical beneficiaries who meet the age and STI/HIV risk eligibility criteria for inclusion are provided a flyer about the study and asked if they would like to receive more information. Those who are interested are offered screening and potential enrollment at the time of their clinic visit or can return to be screened at a later date.
| Study period | ||||||||
|---|---|---|---|---|---|---|---|---|
| Enrollment | Allocation | Post-allocation | ||||||
| Time point | -V1 | 0 | V1 | V2 | M2-6 | V3 | M7-11 | V4 |
| Enrollment | ||||||||
| Eligibility screen | X | |||||||
| Informed consent | X | |||||||
| Allocation | X | |||||||
| Interventions | ||||||||
| Standard STI/HIV prevention counseling | X | X | X | |||||
| KISS behavioral intervention* | X | |||||||
| Monthly SMS/text message† | X | X | ||||||
| STI treatment‡ | X | X | X | |||||
| Referral for HIV management‡ | X | X | X | |||||
| Assessments | ||||||||
| Demographics | X | |||||||
| Sexual Risk Assessment | X | X | X | |||||
| Barriers to STI Care survey | X | X | X | |||||
| STI/HIV Knowledge Assessment | X | X | X | X | ||||
| KISS behavioral intervention feedback form* | X | |||||||
| Pregnancy assessment | X | X | ||||||
| HIV and syphilis testing | Xϕ | X | X | |||||
| NG and CT testing | X | X | X | |||||
| EMR review for STI/HIV diagnoses outside study | X | X | ||||||
Note: * Intervention arm only; † Intervention arm: Prevention Maintenance Intervention (PMI) messages reiterating information received during the EBI session; Control arm: messages with general health information; ‡ If participant diagnosed with testing performed at study visits; ϕ If results from test performed in previous 30 days not available
Table 1: Schedule of enrollment, interventions, and assessments.
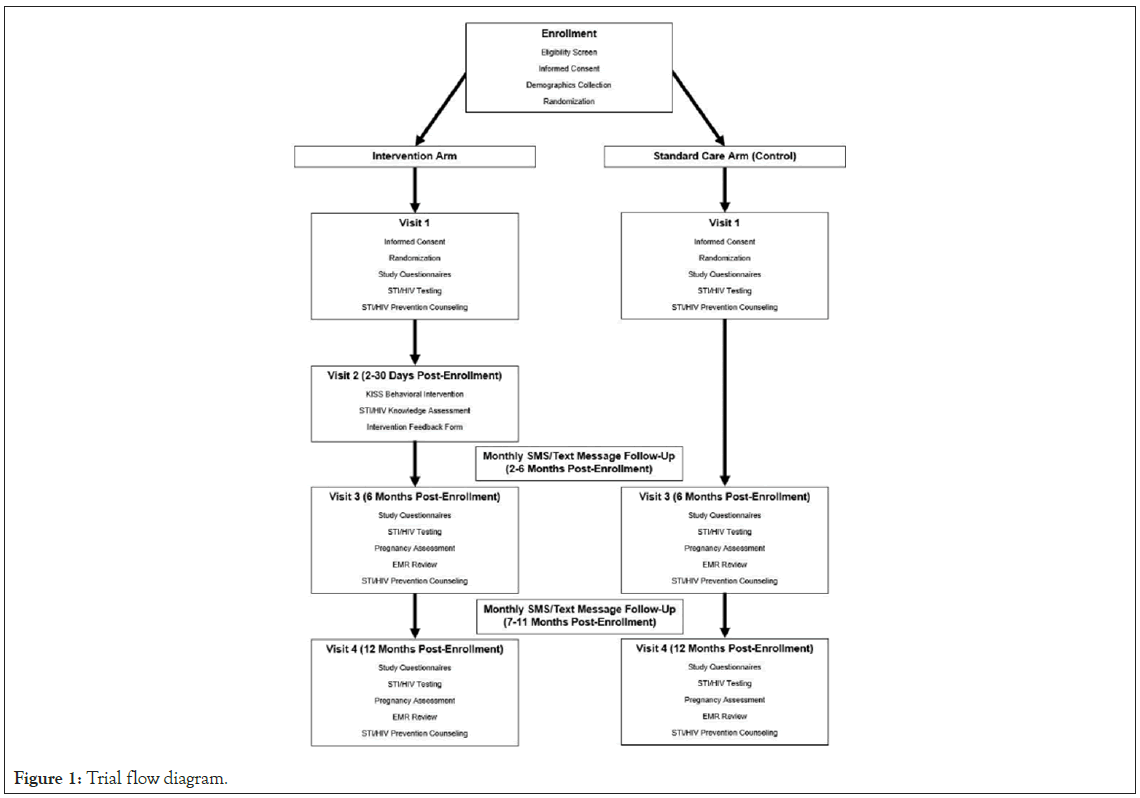
Figure 1: Trial flow diagram.
At enrollment/visit 1, written informed consent is obtained by a study team member, after which participants receive a study ID number and are randomized to the intervention or standard care (control) arm. Randomization is completed at each study site using a permuted block randomization scheme stratified by site with an intervention/control ratio of 1:1. Participants are then asked to provide demographic information to study staff and complete the study questionnaires consisting of a Sexual Risk Assessment (S4), Barriers to STI Care Survey (S5), and an STI/HIV Knowledge Assessment (S6). The study questionnaires are completed on a study tablet or a personal mobile device via the REDCap data management system secure web-based tool. All participants are asked to provide urine, rectal swab, and oropharyngeal swab specimens for NG and CT testing regardless of reported sexual practices. Blood is collected for HIV and syphilis testing. Tests performed within the previous 30 days are not repeated. Participants also receive STI/HIV prevention counseling consisting of routine methods to avoid contracting STIs/HIV and appropriate condom use based on current clinic practice.
Only participants randomized to the intervention arm complete study visit 2 which occurs 2-30 days (+/-30 days) after enrollment and consists of the KISS behavioral intervention delivered in a group setting (more details in Interventions section). Participants are also asked to complete a STI/HIV Knowledge Assessment and a feedback form regarding the intervention at the end of this visit.
All participants complete study visits 3 (6 months after enrollment, +/-30 days) and 4 (12 months after enrollment, +/-30 days) during which participants complete the study questionnaires and provide urine, rectal swab, and oropharyngeal swab specimens for NG and CT testing, and blood for HIV and syphilis serology.
The Electronic Medical Record (EMR) of participants is also be reviewed at these visits to record any incident STIs that occurred between study visits. Participants again receive STI prevention counseling based on current clinic practice at these visits similar to visit 1.
If a participant or their primary partner becomes pregnant or is actively trying to get pregnant, they are invited to return for a final study visit to perform the STI testing and are withdrawn from the study. Study surveys are not performed at this final visit. Participants who are unable to return for this final visit can be withdrawn without performing any additional testing.
If participants are away during the timeframe for visit 3 or visit 4, they are provided with letters of participation in the study which they can take to their local Army clinic to have the STI testing performed. Study questionnaires can be completed via a secure on- line website using a secure emailed password.
Participants diagnosed with an STI at any of the study visits are referred for appropriate treatment as clinically indicated along with prevention counseling based on current clinic practice. Participants diagnosed with HIV are referred to the Preventive Medicine and/or Adult Infectious Disease clinic at MAMC or WAMC, as appropriate for standard counseling and treatment based on current clinical practice and are disenrolled from the study. In addition, all STI diagnoses are entered into the Disease Reporting System-internet (DRSi) as required by Army regulations, and reported to the appropriate state health departments.
Participants receive monthly follow up SMS/text messages during months 2-11 after enrollment, the content of which differs between the two arms. Those in the intervention arm receive Prevention Maintenance Intervention (PMI) messages reiterating information received during the behavioral intervention session. Those in the control arm receive messages containing general health information. If participants are unable to receive text messages sent to mobile phone numbers, monthly messages can be delivered by other means such as email or other messaging applications as identified by the participant.
Compensation
Participants are compensated 25 U.S. Dollars (USD) for blood draws as part of Visits 1, 3, and 4. Participants randomized to the intervention arm are compensated 100 USD along with session-relevant sexual health materials for completing the 2-hour behavioral intervention as part of Visit 2. Participants with children who require childcare in order to complete visit 2 are also offered 50 USD in childcare compensation. Snacks and beverages for consumption are also provided to participants during visit 2. Military service member and federal employee participants need to be on-leave or off-duty to receive compensation, other than compensation for blood draws. In addition to compensation for study visits/blood draws, upon enrollment participants are also given up to five coupons inviting peers to consider enrollment as part of participant referral. If a participant’s peer is eligible to participate and enrolls in the study, the participant who gave the coupon to their peer receives 10 USD. Compensation in this study is made upon completion of individual study visits through a rewards card.
Interventions
The KISS adapted behavioral intervention consists of two components: (1) the group delivered behavioral intervention session and; (2) PMI messages sent to participants via SMS/text messaging.
The first component consists of one 2-hour group delivered intervention session based on Social Cognitive Theory and the Theory of Gender and Power. The intervention is designed to be interactive and foster a sense of pride and social and professional responsibility by emphasizing diverse factors contributing to STI/ HIV risk customized to a military population, including individual factors (STI/HIV risk–reduction knowledge, perceived peer norms supportive of condom use, condom use skills). Each session will consist of at least 2 participants. Sessions are gender specific, however, male participants can be educated by male or female study personnel while female participants are educated by only female study personnel based on the advice of the authors (RD and GW) who developed the intervention and adapted it for use in the military. The KISS adapted behavioral intervention manual is included in the supporting information for reference (S7).
In order to ensure fidelity of implementation of the KISS behavioral intervention and minimize issues with implementation drift, a dual-assurance methodology plan has been implemented wherein participants anonymously rate the health educators after each behavioral intervention group session. In addition, health educators complete a self-assessment after each intervention session which is also anonymous to encourage study staff to honestly assess the implementation of the intervention sessions without fear of reprisal.
The second component of the KISS behavioral intervention consists of monthly follow-up PMI messages reiterating information received during the session sent to participants via SMS/text messaging. The aim of these follow-up PMI messages is to reinforce STI/HIV-prevention knowledge, attitudes and skills acquired in the group sessions. No response to these messages will be required from participants.
Outcome measures
Biological and behavioral primary outcome measures are used in this study to evaluate the efficacy of the KISS adapted behavioral intervention program. Biologic outcomes include incident STIs (CT, NG, and syphilis) as confirmed by laboratory testing during the 12-month study period. Primary behavioral outcomes measured at study visits 3 and 4 include participant-reported proportion of condom use in the preceding 1- and 6-months, number of new sexual partners in the preceding 3- and 6-months, and number of occasions participants had sex while under the influence of alcohol in the preceding 6-months.
Acceptability of the KISS behavioral intervention is assessed by an intervention feedback form completed by participants immediately after the intervention session, which covers satisfaction with the group and whether they would recommend the class to a friend.
Data management
Participant identifying information (name, family member prefix, Department of Defense ID, address, date of birth, telephone numbers, and e-mail address) are electronically recorded on an initial contact information form at the start of the study and are stored a secure, password-protected database with access limited to relevant study staff for follow-up contact and study compensation purposes. Participants are then assigned a participant ID number and all subsequent data collection and follow-up refers to this ID number. A spreadsheet which links individuals with their participant ID number is kept in an encrypted file on a secure computer system.
All study questionnaires and forms are labeled with the participant number and contain no identifying information. Study questionnaires are completed electronically using the REDCap data management system secure web-based tool. Laboratory testing and results are electronically recorded on a laboratory log CRF. All data captured on the study questionnaires, feedback forms, and CRFs during the study are linked only to participant ID numbers and uploaded directly to the U.S. Military HIV Research Program (MHRP) Data Coordinating and Analysis Center (DCAC) secure server in Bethesda, MD using the REDCap data management system. Data is backed up onto encrypted tapes daily. These backup tapes are stored in a secure fireproof cabinet at MHRP offices and then sent offsite weekly. Access to data within REDCap is limited by study, site, and individual user, depending on roles assigned to each user for the study.
Data is entered into the database in accordance with Good Clinical Practices. Data validation and skip patterns are designed into the REDCap application to maximize validity of responses. Additional edit checks are programmed by MHRP DCAC, and run-on data uploaded to the REDCap server. Queries generated by these edit checks are routed to the study coordinator for resolution. A portion (approximately 33%) of CRF data is reviewed by site study staff after data entry to ensure accuracy versus source documentation (medical records) and consistency across study team members.
Biospecimen management
Nucleic Acid Amplification Testing (NAAT) for NG/CT is performed on urine, rectal swab, and oropharyngeal swab specimens per current standard clinical and laboratory protocols at the laboratory where testing is taking place.
Syphilis screening is performed on serum specimens with Treponema pallidum Enzyme Immunoassay (EIA) followed by Rapid Plasma Reagin (RPR) testing for specimens that screen positive per current standard clinical and laboratory protocols at the laboratory where testing is taking place. T. pallidum particle agglutination assay is performed for adjudication of discordant T. pallidum EIA and RPR results.
Serum specimens for HIV testing are shipped to the Center for Disease Detection laboratory in San Antonio, TX (contracting laboratory for Department of Defense) for screening with a fourth- generation antigen/antibody combination HIV-1/2 immunoassay. Specimens that screen positive for HIV are then sent to the WRAIR HIV Diagnostics and Reference Laboratory in Silver Spring, MD for confirmatory testing per their current standard laboratory protocols.
Statistical analysis
Data analysis will be performed jointly between MHRP DCAC and designated study team members.
For the primary outcome of STI incidence, the difference in incidence between the control and intervention groups will be assessed through incident rates and incident density rate at given time points as descriptive statistics. Hazard ratios between treatment and control groups will be modeled for STI incidence.
For the outcomes on condom use, the proportional differences between the control and intervention groups, and the within-group changes from baseline to the end of the study will be assessed by techniques appropriate for proportional difference such as McNemar’s test, Cochran’s Q, or repeated measure ANOVA.
Changes and differences in numbers of sexual partners will be assessed with tests appropriate for continuous outcomes with paired data, such as the paired t-test. In the event that nominal or categorical groups are preferable, alternate analysis such as McNemar’s test or Cochran’s Q may be performed as appropriate for such outcomes.
Acceptability of the intervention will be assessed by participant responses to the feedback form completed at the end of visit 2 in the intervention group using descriptive statistics. Retention will be assessed by the proportion of participants in each group that attend visits 3 and 4 at 6 and 12 months, respectively.
Sub analysis by age, gender and other demographic factors will be conducted. In addition, further comparisons will be made between select dependent variables by univariate analysis. Depending on the outcomes studied, analysis will first involve either simple logistic or linear regression analysis. When more than one independent variable appears to be associated with a significant difference, multiple variables may be evaluated using multivariate analysis.
For missing self-report data on the Sexual Risk Assessment survey at baseline or follow-up visits, data will be analyzed using a list- wise approach including the full data, as well as including multiple imputations to study robustness and sensitivity where viable.
The standard platform for data analysis at MHRP DCAC is SAS® (SAS Institute, Cary, NC), version 9.4. Other software tools may be used if appropriate.
Ethical considerations
All study protocols and amendments to date have been approved by the U.S. Army Regional Health Command-Pacific (RHC-P, lead IRB), the Walter Reed Army Institute of Research (WRIAR) IRB, and the WAMC Human Research Protections Program. Future protocol amendments will be circulated to the site PIs for review and approval, after which they will be submitted to the aforementioned IRBs for approval before implementation. If protocol modifications result in changes to study procedures experienced by participants, they will be informed of the modifications and will be re-consented with an updated consent form. In addition to the aforementioned IRBs, human subjects’ protection representatives of the US Department of Defense (DoD) and the US Army Medical Research and Development Command (USAMRDC) are eligible to review records from this study as part of their responsibility to protect human subjects in research.
Participants may withdraw from study participation at any time by verbal or written communication with the study team. After withdrawal from study participation, data and biological specimens previously collected will continue to be used for study purposes unless the participant notifies the PI that they withdraw consent for such in writing as explained during enrollment in the informed consent process.
Study timeline and status
The study protocol was approved by the RHC-P IRB on 11 March 2020. The current protocol is version number 1.19 dated 08 November 2023. No significant study modifications have been made to the protocol since initial approval. Recruitment began at the JBLM, WA site on 04 November 2020 and the Ft. Liberty, NC site on 19 April 2021. As of 04 August 2023, 155 and 285 participants have been enrolled at the MAMC Preventative Medicine clinic and the WAMC Preventative Medicine clinic, respectively, and the study is ongoing.
The KISS adapted behavioral intervention has demonstrated initial feasibility and acceptability among U.S. Army active-duty personnel and their medical beneficiaries [11]. This study plans to evaluate the efficacy and further evaluate the acceptability of the KISS behavioral intervention in reducing high-risk sexual behaviors and STI/HIV incidence in a similar, but geographically expanded population will inform best-practices and policy regarding STI/ HIV prevention in the U.S. Military population. If effective, the KISS behavioral intervention could fill the void of a much-needed evidence-based intervention to temper the increasing incidence of STI/HIV among the U.S. Military population and contribute to the overall improved health and well-being of U.S. Military servicemembers.
Study results will be shared in peer-reviewed, open-access publications and at national and international research and policy conferences. The final data set will be available upon request to the study team.
We thank the research participants for making this work possible. We would like to express our profound gratitude for the extraordinary efforts of the research team members in ensuring the success of this study: Sheryl A. Bedno, Christine E. Childers, Brooke M. Hernandez, Breanna M. Hernandez, John L. MacArthur, Shakoor Mitchner, Caitlyn R. Patterson.
This work was supported by a Defense Health Program grant (award number: DHP 6.7, DP_67.2_17_I_17_J9_1684) awarded to AK, and a cooperative agreement (W81XWH-18-2-0040) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense. The funders did not and will not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, Department of Defense, or the Henry M. Jackson Foundation for the Advancement of Military Medicine. The investigators have adhered to the policies for protection of human subjects as prescribed in Army Regulation 70–25.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Cebula BR, Walling A, Reynolds A, Yates A, Follen HL, Clark S, et al (2024) Using the Knocking Out Infections through Safer Sex and Screening (KISS) Adapted Behavioral Intervention to Reduce Sexually Transmitted Infections in U.S. Army Medical Beneficiaries: Study Protocol for a Randomized Controlled Trial. J Clin Trials. 14:567.
Received: 01-May-2024, Manuscript No. JCTR-24-31027; Editor assigned: 03-May-2024, Pre QC No. JCTR-24-31027(PQ); Reviewed: 17-May-2024, QC No. JCTR-24-31027; Revised: 24-May-2024, Manuscript No. JCTR-24-31027(R); Published: 31-May-2024 , DOI: 10.35248/2167-0870.24.14.567
Copyright: © 2024 Cebula BR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.