Journal of Oceanography and Marine Research
Open Access
ISSN: 2572-3103
ISSN: 2572-3103
Review Article - (2014) Volume 2, Issue 2
Vanadate(V)-dependent haloperoxidases VHPOs in marine macroalgae catalyze the 2e- oxidation, by H2O2, of bromide and iodide (X-) to X+ species such as HOX, X2 and X3-. Several other substrates, including sulfides, are also oxidized. In the active center, H2VO4- is covalently attached to the Nε of a histidine residue, an arrangement that has successfully been modeled with synthetic analogs of the active center. Vanadium acts as a Lewis acid, forming an intermediate peroxido/hydroperoxido complex. The X+ species generated by the VHPOs halogenates organics present in seawater, from which halomethanes CH4-nXn (n is chiefly 2 or 1) evolve. Additionally, the hypohalous acids can protect the algae against biofilm formation. Prospective applications point towards this biocidal (anti-bacterial) potential of the peroxidases. The seaweeds further exploit the haloperoxidase activity in the synthesis of a multitude of brominated bio-functional molecules. Algal bromoperoxidase activity may account for up to 25% of the atmospheric bromine load. Bromomethanes and iodine released from the aqua- into the atmosphere substantially contribute to ozone depletion, and thus indirectly also to the oxidative conversion of (atmospheric) dimethyl sulfide and nitric oxide.
<Keywords: Vanadate-dependent peroxidases; Macroalgae
With an average concentration of 35 nM, vanadium (essentially present in the form of ion pairs Na+H2VO4-) is the second-to-most abundant transition metal in sea water, outclassed only by molybdenum (present as molybdate MoO42−, ca. 100 nM). Vanadate-dependent haloperoxidases (VHPOs) can be present in marine brown, green and red algae, in (symbiotic) marine cyanobacteria, in Streptomyces bacteria, and in terrestrial fungi and lichen. Since the discovery of the first representative of these haloperoxidases, the bromoperoxidase VBrPO of the brown alga Ascophyllum nodosum [1], also known as knobbed wreck or pig weed, a plethora of macroalgae has been reported to have available VHPOs, hence haloperoxidases that contain vanadate H2VO4− coordinated to a histidine residue in the enzyme’s active center, and thus clearly distinct from the more common hemebased and non-heme peroxidases. Contrasting the heme and non-heme peroxidases, VHPOs are rather robust: they withstand comparatively high concentrations of hydrogen peroxide and temperatures up to 70°C, and are also resistant to high concentrations of organic solvents [2]. Depending on the substrate selectivity, one distinguishes between VHPOs that catalyze the oxidation of (i) iodide only (VIPOs), (ii) iodide and bromide (VBrPOs), and (iii) iodide, bromide and chloride (VClPOs). Along with halides, pseudohalides (such as cyanide and thiocyanate) and sulfides (for example dimethylsulfide) can be oxidized. Prochiral sulfides RR’S are oxidized enantioselectively. The general reactions for the enzymatic two-electron oxidation of a substrate X− (halide(1-) and pseudohalide(1-)) or a (prochiral) sulfide are depicted in Eqs. (1a) and (1b), respectively. In addition to halides and sulfides, VHPOs catalyze the oxidation of many other organic substrates either directly or indirectly, i.e. via intermittent bromination. In the absence of a substrate, singlet oxygen is formed, Eq. (1c).
X- + H2O2 + H+ → HO–X + H2O (1a)
RR’S + H2O2 → RR’S=O + H2O (1b)
HOX + H2O2 → 1O2 + H2O + HX (1c)
Chloroperoxidases are mostly present in Streptomyces bacteria [3,4] and in terrestrial fungi where they serve as chlorinating agents for antibiotic compounds (Streptomyces), and for the production of hypochlorous acid HOCl (fungi). HOCl degrades the cell wall lignocellulose and thus allows access of the fungal hypha to the intracellular space [5]. An iodoperoxidase has recently been found in the cyanobacterium Acaryochloris marina, a symbiotic organism isolated from the sea squirt Lissoclinum patella collected from the Palau Island in the Pacific [6]. Apart from having available a VIPO, A. marina is also of interest in as far as it contains chlorophyll-d (also present in red algae), and hence a chlorophyll allowing for the absorption in the near infrared [7].
At a mean salt concentration of 0.6 M, the pKa for the equilibrium H2VO4 − / HVO4 2− + H+ is 7.95 [8]. At a pH of ~8, free vanadate is thus available in the form of a mix of mono- and dihydrogenvanadate, HVO4 2− and H2VO4 −. Along with vanadate(V), some vanadate(IV) H3VO4 − ≡ VO(OH)3 − can be present, formed by one-electron reduction from vanadate(V) through biological as well as non-biological activity, and via the desorption from superficial sediments in hypoxic bottom waters [9]. The mean mean concentrations of the VHPOs’ substrate halides, c(X−), are 0.55 M (X = Cl), 0.82 mM (X = Br), and 0.47 μM (X = I). Kelps, such as the brown alga Laminaria digitata, known and utilized for its capacity to accumulate iodide, take up iodide from seawater by means of the oxidation of I− with H2O2 to HOI, catalyzed by a VIPO. Within the cells, HOI is reduced back to I− [10], where it is present in the extracellular matrix, associated via hydrogen bonds with organic molecules, in concentrations of up to 50 mM [11]. The concentration of H2O2 in sea water is rather variable; in open ocean water, c(H2O2) can go up to 0.25 μM. H2O2 is also produced biogenically in the frame of metabolic processes; VHPOs which, in contrast to the heme peroxidases, are particularly robust against H2O2, thus (also) counteract oxidative stress [12].
This review provides a brief overview of structural and functional details of algal VHPOs, including mechanistic aspects of substrate oxidation, and also including the relationship of VHPOs to phosphatases, and hence the vanadate-phosphate antagonism. Synthetic mimics of the VHPOs will be briefly discussed with special reference to their (potential) use as anti-fouling agents and thus protective capability for stationary and mobile submerged structures, such as hulks. Finally, the impact of the algal production of inorganic halogens, and of halogenated organic compounds, on the marine and atmospheric environment, and thus also – in the context of ozone depletion – the global radiation budget – will be addressed.
Examples for marine macroalgae containing bromoperoxidases are the brown algae Ascophyllum nodosum, Macrocystis pyrifera, Laminaria digitata and Fucus distichus, the red algae Corallina officinalis and C. pilulifera, and the green algae Ulva lactuca and Halimeda sp. (Figure 1). Information on the active site structure is available for the bromoperoxidases of A. nodosum [13] and C. pilulifera [14], and for the phosphate variants (i.e. the apo-enzyme reconstituted with phosphate) of C. pilulifera and C. officinalis [15]. In the case of A. nodosum, two isoenzymes have been structurally characterized, viz. VBrPO(AnI) [13] and – by the group of Hartung – VBrPO(AnII) [16]. VBrPO(AnI) is a homodimer (557 amino acids per subunit) of molecular mass M = 136 kDa, while VBrPO(AnII) is a hexamer (641 amino acids) of M = 420 kDa. The two isoenzymes share 41% sequence homology. The peroxidase from C. pilulifera, VBrPO(Cp), is a homododecamer (hexamer of homodimers) with 595 amino acids per subunit, stabilized by one Ca2+ per subunit.
Figure 2 provides insight into structural details of the active centers of VBrPO(AnII) and VBrPO(Cp)+ bromide. In both peroxidases, active site vanadate(V), commonly present in the form of HnVO4 (3-n)− (where n probably equals 2), is coordinated to the Nε of a histidine residue of the protein matrix, giving rise to a trigonal bipyramidal coordination environment for the vanadium center, with the histidine Nε and an oxido(1-)/hydroxido ligand in the axial positions. For the first and second sphere environment of vanadium in VBrPO(AnI) see also the schematic presentation in Figure 3a and 3b. Noteworthy is a near-by serine (Ser450 in the case of VBrPO(AnII)) that might mediate the contact to the catalytic center via the intermittent formation of bromoalanine, Eq. (2) [17]. The bromide in the active center of the VBrPO(Cp) is located in-between vanadate and an active site arginine (Arg387) within hydrogen bonding distances [14], suggesting the activation of bromide by arginine. The absence of direct binding of bromide to vanadium has also been evidenced by V-XAS studies [18].
Figure 2: The active centre of the vanadate-dependent bromoperoxidases VBrPO(AnII) (left [16]) and the VBrPO(Cp) (right [14]), the latter soaked with bromide (cyan sphere). Histidine (His517 in the case of VBrPO(AnII)) is coordinated to the vanadium center (yellow) of the vanadate anion (oxygens in red) via Nε. Reproduced with permission from Elsevier.
–CH2OH + Br− + H+ → –CH2Br + H2O (2)
Interestingly, there is a close homology between the active site arrangements of phosphatases and VHPOs [19,20]. Given the structural similarity between the tetrahedral anions phosphate and vanadate, such a homology might be anticipated. There are, however, clear-cut differences between phosphate and vanadate with respect to the redox activity and the potential expansion of the coordination sphere: Vanadate(V) is easily reduced to oxidovanadium(IV) while phosphate is comparably redox-stable. Further, vanadium can easily adopt the coordination number five (as in the VHPOs) – the reason for the inhibitory effect of vanadate towards many phosphatases – while penta-coordinate phosphate is restricted to enzymes’ transition state. In Figure 3, the coordination environments of VBrPO (a) and rat acid phosphatase reconstituted with vanadate (b) are compared, clearly demonstrating the similarities. Even more so, vanadatesubstituted bacterial acid phosphatases have been shown to exhibit bromoperoxidase as well as sulfide peroxidase activity [21].
The primary species obtained from the enzymatic oxidation of bromide with hydrogen peroxide according to Eq. (1a) (X = Br) is hypobromite that, released into water of pH ~8, is mainly present as hypobromous acid HOBr: the pKa for the equilibrium HOBr ⇆ H+ + –OBr is 8.7. In secondary reactions, HOBr undergoes symproportionation with bromide to form bromine, Eq. (3a), which further can take up with Br– and generate tribromide, Eq. (3b). Only Br2 is sufficiently electrophilic for the formation of a C–Br bond in bromination reactions [22].
HOBr + Br− → Br2 (3a)
Br2 + Br− → Br3− (3b)
The initiating step in the oxidation of bromide (and other substrates of the peroxidases) with H2O2 is the coordination – and thus activation – of peroxide O2 2− to the vanadium center, (a) in Scheme 1. Concomitantly, the coordination geometry changes from trigonal-bipyramidal towards strongly distorted tetragonal-pyramidal, stabilized by hydrogen bonding interaction, mediated via H2O, between the apical oxido(2-) ligand and the apical histidine [23]. The next step is the protonation of the peroxido ligand to form a hydroperoxido intermediate [24], (b) in Scheme 1. Nucleophilic attack of bromide onto the peroxido oxygen (c) delivers hypobromous acid (e), likely via a hypobromito intermediate, (d) [25]. The oxidation of sulfides to sulfoxides, Eqn. (1b), likely proceeds accordingly, i.e. by nucleophilic attack of the sulfide to the peroxo oxygen of the hydroperoxido intermediate (f), followed by the release of the sulfoxide, (g) in Figure 4. The chiral information for the generation of chiral sulfoxides RR’S=O is communicated by the protein matrix in the immediate proximity of the reaction center [8].
The net halogenation reaction catalyzed by VHPOs is represented by Eq. (4). Generally, basic inorganic vanadium (V) compounds such as [VO2(H2O)4]+ and H2VO4 − also catalyze the oxidation of bromide by peroxide and thus, in a successive step, the bromination of organic substrates, with low turn-over numbers, however, and commonly at non-physiological conditions. Several synthetic mimics for the active center of the VBrPO have been synthesized that are efficient catalysts in the peroxide-driven bromination of organic substrates and/or the enantio-selective oxidation of organic sulfides. Two examples are shown in Figure 4.
X- + H2O2 + RH + H+ → R–X + 2H2O (4)
Natural products formed in the wake of the catalytic activity of haloperoxidases in marine macroalgae – VBrPOs in red algae in particular – encompass simple compounds such as CH2Br2 and CHBr3, to be addressed in the context of the aquatic and tropospheric halogen chemistry in section 4, mixed halomethanes, for example CHBr2I and CHBr2Cl, and more complex organic compounds, a selection of which is collated in Figure 5. This selection includes compounds such as α-snyderol, formed by bromination of the sesquiterpene (E)-(+) nerolidol [5,26] (5 in Figure 5), and (E)-deacetyllaurencin (6 in Figure 5). Compound 6 forms from laurediol by cyclisation plus bromination, employing a VBrPO present in the red alga Laurencia nipponica [27].
As noted, the hypohalous acids generated by marine macroalgae do not only initiate a wide array of halogenations and – more generally – oxidations of organic substrates, but also protect the sea weeds against bacterial infection in as far as they oxidatively damage the cellular membrane of bacteria and thus cause leakage of intracellular components, or disrupt the bacterial signaling system and/or gene expression, for example by brominating lactones and/or furanones responsible for regulatory functions [28]. The bromofuranone (7 in Figure 5) is an example. These antibacterial and thus antifouling characteristics of VHPOs can, in principle, also be exploited for the disinfection of medicinal equipment [29], and the destruction of underwater biofilms. An example for the latter is the protection of submerged structures such as the paintings of ship’s hulls [30]. The antimicrobial effect of VClPOs does further have potentiality in endodontics, i.e. in the treatment of dental biofilms produced by Streptococcus mutans [31] and Enterococcus faecalis [32]. In the context of these anti-microbial effects, it is interesting to note that allelopathic activity has been observed for the marine benthic diatom Nitzschia pellucida. This organism releases cyanogen bromide BrCN that causes cell death of other co-occuring competing diatoms, and there is clear evidence that this biogenic production of BrCN is connected to haloperoxidase activity [33].
The capability of VHPOs to catalyze the oxidation of prochiral sulfides to chiral sulfoxides, Eq. (1b), hints towards a possible application also in a pharmaceutical context [34,35]. Further, the selection of organic compounds in Figure 5, naturally produced by oxidative halogenation as catalyzed by algal VHPOs, VBrPOs in particular, clearly demonstrates the potential of these peroxidases also in industrial applications. An example is the formation of 3-cyanopropanonic acid – a key intermediate in the synthesis of acrylonitrile (and thus in the manufacture of polyacrylonitrile) – by oxidative decarboxylation of glutamic acid with H2O2 in the presence of VClPO and catalytic amounts of bromide [36], as shown in the (non-stoichiometric) Eq. (5). Glutamic acid is abundant in a number of biofuel rest streams.
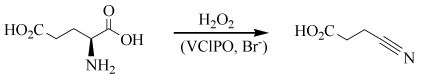 (5)
(5)
A graphical overview of the speciation of atmospheric components through the interaction with (biogenic) halogen compounds – exemplified for bromine – released by seaweeds and phytoplankton from essentially marine environments into the atmosphere is provided in Figure 6. It should be noted that there is also substantial anthropogenic input of methylbromide into the environment: CH3Br is a widely employed gaseous pesticide. Bromomethanes are further released by sediments, partially via bacterial degradation. In the following, selected scenarios will briefly be addressed
Figure 6: Some aspects of the cycling of halogen species, exemplified here for bromine, between the hydrosphere (blue) and the atmosphere, and within the atmosphere. [VBrPO] is vanadate-dependent bromoperoxidase, [HPO] represents a yet to be identified haloperoxidase. {CH} stands for more or less complex dissolved organic matter, mediating the generation of bromomethanes via the intermediate formation of the partially labile brominated organics {CBr}.
In these scenarios, halogenated methanes play a pivotal role. While mono-halogenated methane CH3X (X = Br, I) is essentially formed by transfer of the methyl group of adenosyl-S-methylmethionine AdMetS+CH3 to the halide [10], Scheme 2, hence without the participation of VBrPO, the generation of di- and tri-halogenated methanes CH2X2 and CHX3 is dominated by haloperoxidases associated with algae and cyanobacteria. These organisms resort to halide and H2O2 (produced by photosynthesis, photorespiration, or exogenously), or to ozone O3 when the algae are exposed to air at low tide. The reaction with external halide is facilitated by the fact that, in many cases, the algal VHPOs are located in the transitional region between cortex and medulla of the algal cells forming the outer cell wall. The primary step in the formation of CHX3 is the oxidation of X− to an {X+} species such as HOX, X2 or X3 −, followed by the halogenation of dissolved organic matter {CnH} that (partially) decays, releasing the haloform [28,37]. For the generation of bromoform CHBr3, see the non-stoichiometric reaction sequence provided in Eq. (6). Iodoform CHI3 can form correspondingly [38]. Estimates of the global coastal sea-to-air fluxes of bromine in the form of brominated methanes CH4-xBrx amount to 2.5.109 mol (2.108 kg) Br per year [39], accounting for about 25% of tropospheric + lower stratospheric ozone depletion by bromine that is released photolytically from CH4-xBrx, see Eq. (7) for x = 2. Coastal and equatorial regions represent regional hotspots of bromocarbons. As an example, the sea-surface water concentration of bromoform in the Gulf of Mexico can go up to 1.7 nM [40].
Br → {Br+} + e− (6a)
{Br+} + {CnH} → {CnBr} + H+ (6b)
{CnBr} → {Cn-1} + CHBr3 (6c)
CH2Br2 + hν → {CH2Br} + Br (7a)
Br + O3 → BrO + O2 (7b)
BrO + O3 → Br + 2O2 (7c)
A particularly efficient mechanism of ozone depletion during spring time in polar regions involves hypobromous acid HOBr absorbed in liquid brine particles: HOBr and bromide symproportionate to bromine Br2, Eq. (8a), from which atomic Br is generated photolytically, Eq. (8b). As shown in Eq. (7b), Br reacts with ozone to form BrO. HOBr is then recovered in the reaction with the radical HO2, Eq. (8c), generated for example from O2, hydroxy radicals and carbon monoxide, Eq. (9). The reaction sequence (8a) to (8c) proceeds auto-catalytically and is therefore sometimes referred to as ‘bromine explosion’ [41].
HOBr + Br− + H+ → Br2 + H2O (8a)
Br2 + hν → 2Br (8b)
BrO + HO2 → HOBr + O2 (8c)
O2 + OH + CO → HO2 + CO2 (8d)
In coastal regions, kelps dominate the iodine flux from the ocean to the atmosphere by converting, in connection with oxidative bursts, iodide to iodocarbons and to IO, thus providing precursors for cloud condensation. Iodide is an effective scavenger for Reactive Oxygen Species (ROS) such as superoxide (O2 −) and singlet oxygen (1O2), H2O2 in the cell wall space, and external ozone, Eq. (10a). The reaction with ozone in particular effectively impacts atmospheric chemistry [11]. Similarly, iodine formed according to Eq. (10a), by oxidation of iodide with ROS and released into the atmosphere, or via photo-dissociation of methyl iodide, Eq. (10b), can react with ambient ozone to form iodine oxide, Eq. (10c) [42].
O3 + 2I− + 2H+ → I2 + H2O + O2 (10a)
CH3I + hn → {CH3} + I (10b)
I + O3 → IO + O2 (10c)
The capability of atomic and molecular halogens, as well as of halogen oxides and hypohalous acids, to oxidize substrates can also result in the oxidative conversion of dimethyl sulfide (CH3)2S and elemental mercury. (CH3)2S is released from the marine environment into the atmosphere by dimethylsulfoniopropionate DMSP [43]; DMSP derives from S-methylmethionine), and subjected to oxidation to dimethylsulfoxide (which is further processed to sulfurous and sulphuric acid). BrO, formed according to Eq. (7b), can act as the oxidant, Eq. (11). The atmospheric depletion of elemental mercury can be achieved via the oxidation of Hg with bromine, Eq. (12). Elemental mercury is released into the troposphere by volcanic events, anthropogenic issues, and volatilization – by methylation – of marine CH3HgCl with subsequent photolytic fission of (CH3)2Hg, Eq. (13). Finally, halogen species such as BrO and IO exert a pronounced impact onto the speciation of atmospheric nitrogen oxides [41]; for examples see Eqs. (14) and (15).
(CH3)2S + BrO → (CH3)2SO + Br (11)
Hg + Br → ½Hg2Br2; ½Hg2Br2+ Br → H (12)
CH3HgCl + {CH3} → (CH3)2Hg + {Cl}, (CH3)2Hg + hν → Hg + C2H6 (13)
BrO + NO Br + NO2; NO2 + hν → NO + O (14)
IO + NO2 → IONO2; IONO2 + hν → I + NO3 (15)
Inorganic vanadium(V) compounds are versatile oxidation catalysts. In common applications in homogenous and heterogeneous catalysis, their catalytic activity is due to the ease of change between the oxidation states +V and +IV [44]. In contrast, the peroxidase activity of the algal peroxidases (as well as of peroxidases from diatoms, bacteria, fungi and lichen) relies on the Lewis acidity of the V5+ center. This fact should revive and initiate novel studies into the catalytic potentiality of high-valent, comparatively simple vanadium compounds. Also of interest in this context are the anti-fouling properties of nanoparticulate V2O5 [45], reminiscent of the antibacterial and anti-fungal potential of VHPOs present in seaweeds and diatoms. The antibacterial properties of externally applied native VHPOs or, even more efficient, in the form of mutants thereof [46], will likely have an impact on the future development of formulations for disinfection, for example of submerged structures, but also in a medicinal context.
In addition, a more detailed study of the reaction paths leading to the production of the partially rather complex halogenated organics [47,48] should provide more profound insight into the mode of action of the peroxidases, as well as into the versatile synthetic potential of the VHPOs. A more broader level of awareness on functional aspects will encourage synthetic chemists to pursue the synthesis of novel inorganic and metal-organic mimics of the active center, in particular complexes with the oxidovanadium(V) moiety in a five-coordinate environment .
The release of volatile organohalides, bromo- and iodomethanes in particular, into the troposphere, and further into the stratosphere, has provided valuable – but still incomplete – information on the role of halogens in atmospheric processes (including ozone depletion) in the past few years [11,41]. The recent detection of BrCN as a product of haloperoxidase activity [33] is another encouraging record pointing into this direction. Interest in view of the role of haloperoxidases further arises from these enzymes’ involvement in the speciation and distribution of halides from anthropogenic sources. An auspicious example in this respect is radioactive iodine-129 [42], a fission product from nuclear weapon tests, and thus of particular environmental concern.