Research Article - (2018) Volume 4, Issue 1
Variations in the Functional Properties of Soybean Flour Fermented with Lactic Acid Bacteria (LAB)-Consortium
*Corresponding Author: Ogodo AC, Department of Microbiology, Faculty of Pure and Applied Sciences, Federal University Wukari, P.M.B. 1020 Wukari, Taraba State, Nigeria Email:
Abstract
The functional properties of soybean flour fermented with lactic acid (LAB)-consortium was evaluated. Soybean was processed into flour, fermented spontaneously and with LAB-consortium previously isolated from maize (Lactobacillus plantarum WCFS1+Lactobacillus rhamnosus GG, ATCC 53/03+Lactobacillus nantensis LP33+Lactobacillus fermentum CIP 102980+Lactobacillus reuteri DSM 20016) and sorghum (Pediococcus acidilactici DSM 20284+Lactobacillus fermentum CIP 102980+Lactobacillus brevis ATCC 14869+Lactobacillus nantensis LP33+Lactobacillus plantarum WCFS1) to evaluate their effects on the functional properties of the flour at 12 h intervals using standard techniques. The result shows gradual decrease in bulk density with increasing fermentation period ranging from 0.74 ± 0.03 g/mL to 0.72 ± 0.03 g/mL (natural), from 0.74 ± 0.03 g/mL to 0.70 ± 0.02 g/mL (LAB-consortium from maize) and from 0.74 ± 0.03 g/mL to 0.70 ± 0.02 g/mL (LAB-consortium from sorghum) fermentation. The swelling capacity decreased from 0.77 ± 0.03 g/mL to 0.64 ± 0.01 g/mL, from 0.77 ± 0.03 g/mL to 0.59 ± 0.01 g/mL and from 0.77 ± 0.03 g/mL to 0.61 ± 0.03 g/mL in natural, LAB-consortium from maize and sorghum fermentations respectively. Water holding capacity decreased from 2.4 ± 0.03 mL/g to 1.9 ± 0.03 mL/g, from 2.4 ± 0.03 mL/g to 2.0 ± 0.03 mL/g and from 2.4 ± 0.03 mL/g to 1.9 ± 0.03 mL/g in natural, LAB-consortium from maize and sorghum fermentation respectively. Oil holding capacity increased significantly (p<0.05) with increasing fermentation time, ranging traditional bread and 0.62-0.69 g/mL reported by Igbabul et al. on
fermented mahogany beansfrom 8.92 ± 0.02 mL/g to 9.30 ± 0.03 mL/g (natural), 8.92 ± 0.01 mL/g to 9.63 ± 0.03 mL/g (LAB-consortium from maize) and from 8.92 ± 0.03 mL/g to 9.69 ± 0.03 mL/g (LAB-consortium from sorghum) fermentations. The least gelation concentration ranged from 3.0% (unfermented) to 6.0% (other fermentation products). Emulsion capacity (EC) increased from 35.88 ± 3.12% to 44.33 ± 1.33%, from 35.88 ± 3.12% to 46.83 ± 3.18% and from 35.88 ± 3.12 % to 45.99 ± 2.21% in natural, LAB-consortium from maize and sorghum fermentations respectively. This suggests the potentials of LAB-consortia fermentation in improving nutritional and functional properties of soybean flour.
Keywords: Functional Properties, Soybean flour, Fermentation, LAB-consortium
Introduction
In developing countries of Africa like Nigeria, malnutrition in terms of protein is prevalent due to inadequate or high cost of meat and milk, hence the need for alternative sources of protein [1]. Legumes such as Bambara groundnut pigeon pea, soybeans are nutritious foods which can serve as an alternative to animal proteins owing to their reported nutritional attributes [2,3]. Therefore, legume seeds flours can be employed in food formulations to supplement proteins and reduce over dependent on animal protein if they contain the desired functional properties. However, the utilization of legumes has been greatly affected by the presence of anti-nutritional factors such as phytate, tannins, oxalates, polyphenols and enzyme inhibitors. Although treatments such as heat, germination, soaking and fermentation have been reported to reduce the anti-nutritional factors in food products [4-8].
Soybean (Glycine max) was first grown in Eastern Asia. It has long been used as important sources of protein, complementing grain proteins, in Asian countries [9]. Fermented soybean products has been reported to contain functional components such as peptides, isoflavonoids and consumption of soybeans and soy foods is associated with lowered risks for several cancers including breast, prostate, colon, cardiovascular diseases and improves bone health [10]. Adelakun et al. reported that the protein content of soybean is 32% to 42%, depending on the variety and growth conditions, of which approximately 80% is composed of 2 storage globulins, 7S globulin (β-conglycinin) and globulin (glycinin), having various functional and physicochemical properties [9].
Fermentation is a major process used in the production of foods from soybeans [10]. Fermentation leads to changes the physicochemical, organoleptic properties (colour, flavour) and active components of soybean products [11]. Changes to phenolic composition of legumes brought about by fermentation have been reported to significantly increase free radical scavenging capacity of legumes [12,13]. Major microorganisms implicated in soybean fermentation are filamentous fungi, Bacillus species and lactic acid bacteria [11]. It has been reported that soybeans fermented with filamentous fungi enhanced the phenolic content, radical scavenging activity and antioxidant activity in fermented product [14,15].
Lactic acid fermentation is a common way of preparing food such as maize porridge, alcoholic beverages and dairy products traditionally in many African countries [16,17]. Fermentation by lactic acid bacteria increases food palatability, proteins, vitamins and as well confers preservative and detoxifying effect on food. Also, lactic acid bacteria fermented foods boost immunity against bacterial ds [18-20]. Beta glycosides are known to be hydrolyzed by Some Lactobacilli and Bifidobacteria thereby enhancing the bioavailability of isoflavones contained in soybean by fermentation [21]. Therefore, the present study evaluates the effect of lactic acid bacteria consortium fermentation on the functional properties of soybean flour.
Materials and Methods
Source of materials
Cream coloured soybean (Glycine max (L) Merr.) was bought from Yaba market of Lagos, Lagos State, Nigeria and transported to Federal Institute of Industrial Research Oshodi (FIIRO) where it was identified and analysed. Lactic acid bacteria (Lactobacillus plantarum WCFS1, Lactobacillus rhamnosus GG, ATCC 53/03, Lactobacillus nantensis LP33, Lactobacillus fermentum CIP 102980, Lactobacillus reuteri DSM 20016, Pediococcus acidilactici DSM 20284, Lactobacillus fermentum CIP 102980 and Lactobacillus brevis ATCC 14869) used were stock previously isolated from fermenting maize and sorghum.
Sample preparation
The raw seeds of soybeans were freed of foreign materials, washed, rinsed with distilled water and then dried with hot air oven (GL, England) at 60°C for 8 h. The dried samples were milled into powder using milling machine (CNC, Germany) disinfected with 70% ethanol. The flour was stored in sterile air tight containers at 4°C for further use [22].
Choice of starter
The Lactic acid bacteria consortium were selected based on their tolerance to acid, salt, lowering of pH, level of acid production and growth on nutrient depleted medium after a pre-fermentation studies.
Inoculum preparation
The starter culture was prepared following the method of [22] as described by [17] with slight modification. Each of the lactic acid bacteria obtained as stock previously isolated from maize and sorghum was grown at 37°C for 24 h on autoclaved MRS broth in test-tubes of 10 ml aliquots. Then five lactic acid bacteria were combined as follows, Lactobacillus plantarum WCFS1+Lactobacillus rhamnosus GG, ATCC 53/03+Lactobacillus nantensis LP33+Lactobacillus fermentum CIP 102980+Lactobacillus reuteri DSM 20016, for consortium from maize; and Pediococcus acidilactici DSM 20284+Lactobacillus fermentum CIP 102980+Lactobacillus brevis ATCC 14869+Lactobacillus nantensis LP33+Lactobacillus plantarum WCFS1, for consortium from sorghum respectively. These were grown in a 250 ml Erlenmeyer flasks containing 210 ml MRS broth respectively, and incubated for 48 h at 37°C for the inoculum to build-up. The inocula were harvested by centrifugation and washed with sterile distilled water at 5000 rpm for 10 min. The washed cells were diluted using sterile distilled water to obtain 0.5 McFarland, standard of 1.0 × 108 cells/mL.
Fermentation procedure of soybean flour
Fermentation was carried out following a modification of the method described by [17] with slight modification. 500 g of the raw flour was mixed with sterile distilled water in the ratio of 1:2 w/v in sterile fermentation containers. 0.5 g/L potassium sorbate was added (to inhibit fungal growth and other contaminating organisms) except the set up for spontaneous fermentation. The mixture was inoculated with 10 ml of 1.0 × 108 cells/mL of the lactic acid bacteria consortium suspensions and allowed to ferment. Starter culture was not added to one of the set-ups to allow for spontaneous fermentation. Samples were taken at 12 h intervals for analysis of the functional properties.
Determination of functional properties of fermented soybean flour
The bulk density of the fermented soybean flour was determined using the method of [23]. The water and oil holding capacities were determined according to the method described by [6]. The method of [24] was used to determine the swelling capacity of the soybean flour. The least gelation concentration of the flour was determined with the method described by [25] while the emulsion capacity was determined using the method described by [26].
Results
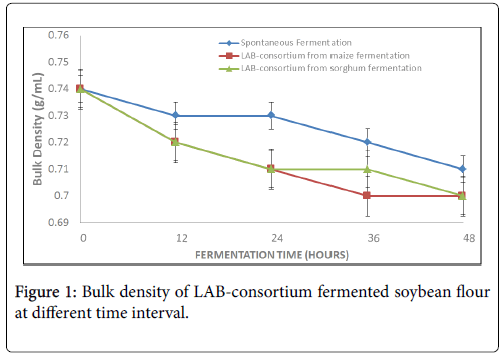
The bulk density decreased gradually as fermentation time increased and ranged from 0.74 ± 0.03 g/mL to 0.72 ± 0.03 g/mL (natural fermentation), 0.74 ± 0.03 g/mL to 0.70 ± 0.02 g/mL (LAB-consortium from maize fermentation) and from 0.74 ± 0.03 g/mL to 0.70 ± 0.02 g/mL (LAB-consortium from sorghum) fermentation (Figure 1).
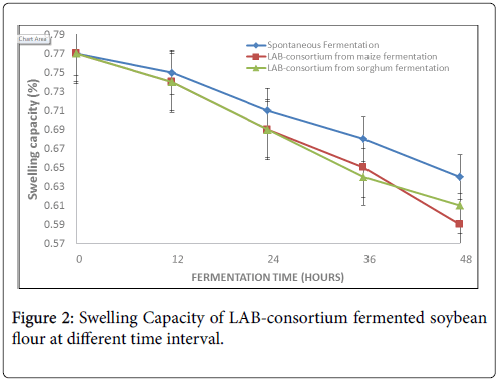
Figure 2 presents the swelling capacity of the fermented soybean flour which showed a gradual decrease with increasing time of fermentation. In natural fermentation, it ranged from 0.77 ± 0.03% to 0.64 ± 0.01%, from 0.77 ± 0.03% to 0.59 ± 0.01% and from 0.77 ± 0.03% to 0.61 ± 0.03% in natural, LAB-consortium from maize and sorghum fermentations respectively.
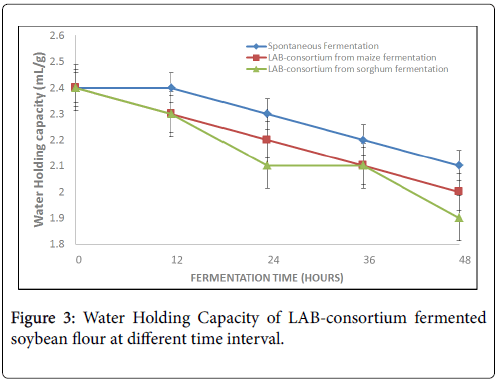
The result of water holding capacity of the fermented flour decreased with increasing time of fermentation from 2.4 ± 0.03 mL/g to 1.9 ± 0.03 mL/g, from 2.4 ± 0.03 mL/g to 2.0 ± 0.03 mL/g and from 2.4 ± 0.03 mL/g to 1.9 ± 0.03 mL/g in natural, LAB-consortium from maize and sorghum fermentation respectively (Figure 3).
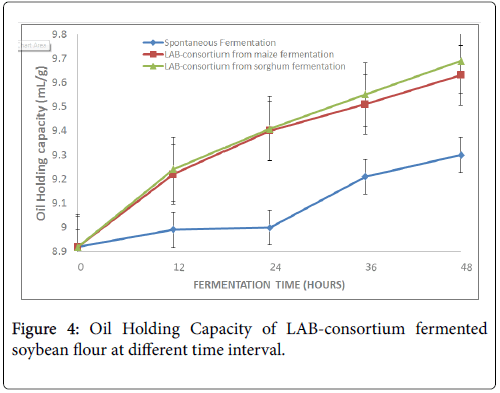
Oil holding capacity increased significantly (p<0.05) with increasing fermentation time ranging from 8.92 ± 0.02 mL/g to 9.30 ± 0.03 mL/g (natural), 8.92 ± 0.01 mL/g to 9.63 ± 0.03 mL/g (LAB-consortium from maize) and from 8.92 ± 0.03 mL/g to 9.69 ± 0.03 mL/g (LABconsortium from sorghum) fermentations (Figure 4).
The result of the least gelation concentration of the fermented flour is presented in Table 1 and ranged from 3.0% (unfermented) to 6.0% (other fermentation products). The lowest was observed in the unfermented sample while the highest was observed at the 36 and 48 h of the fermented products.
| Concentration (%) | 0 h | 12 h | 24 h | 36 h | 48 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F* | NF | MF | SF | NF | MF | SF | NF | MF | SF | NF | MF | SF | |
| 1.0 | V | V | V | V | V | V | V | V | V | V | V | V | V |
| 2.0 | V | V | V | V | V | V | V | V | V | V | V | V | V |
| 3.0 | G | V | V | V | V | V | V | V | V | V | V | V | V |
| 4.0 | G | G | G | G | V | V | V | V | V | V | V | V | V |
| 5.0 | G | G | G | G | G | G | G | V | G | G | V | V | V |
| 6.0 | G | G | G | G | G | G | G | G | G | G | G | G | G |
| 7.0 | G | G | G | G | G | G | G | G | G | G | G | G | G |
| 8.0 | G | G | G | G | G | G | G | G | G | G | G | G | G |
| 9.0 | G | G | G | G | G | G | G | G | G | G | G | G | G |
| 10.0 | G | G | G | G | G | G | G | G | G | G | G | G | G |
| LGC | 3.0a | 4.0a,b | 4.0a,b | 4.0a,b | 5.0b,c | 5.0b,c | 5.0b,c | 6.0c,d | 5.0b,c | 5.0b,c | 6.0c,d | 6.0c,d | 6.0c,d |
F*=Unfermented sample; NF=naturally fermented; MF=fermented with LAB consortium from maize; SF=fermented with LAB consortium from sorghum; V=viscous; G=gel; LGC=Least gelation concentration in percentage.
Values with the same superscript are not significantly different (P>0.05)
Table 1: Least gelation concentration (LGC) (%) of LAB-consortium fermented soybean flour.
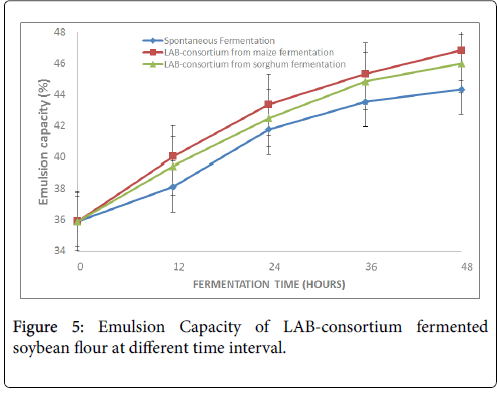
Figure 5 presents the emulsion capacity (EC) of the fermented soybean flour. The result showed a significant increase (P<0.05) in the EC as fermentation time progressed. The increase ranged from 35.88 ± 3.12% to 44.33 ± 1.33%, from 35.88 ± 3.12% to 46.83 ± 3.18% and from 35.88 ± 3.12% to 45.99 ± 2.21% in natural, LAB-consortium from maize and sorghum fermentations respectively.
Discussion
The bulk density of the fermented flour in the present study decreased with increasing fermentation period. It ranged from 0.74 ± 0.03 g/mL (raw sample) to 0.70 ± 0.02 g/mL (48 h LAB consortium from maize and sorghum fermentations). This observation is in agreement with the report of other researchers on similar legumes. For instance, Adebowale and Maliki reported decrease in bulk density of fermented pigeon pea flour in the range of 0.80 to 0.63 g/mL [2]. Buta & Emire reported a decrease in the bulk density of quality protein maize (QPM)-soybean blend after 48 h fermentation which was observed to be consistent with the report of Lalude & Fashakin on a soybean based weaning food [27,28]. The values in the present study is also comparable with 0.68 reported by Mesfin and Shimelis on the effect of soybean/cassava on the proximate composition of Ethiopian traditional bread and 0.62-0.69 g/mL reported by Igbabul et al. on fermented mahogany beans (Afzelia africana ) flour [29,30]. The reduction in the density as a result of fermentation is an advantage when the flour are to be used for the formulation of infant foods since fermentation has been reported by Singh et al. to be a traditional means of preparing low density foods [6].
In the present study, the swelling capacity (SC) decreased with increasing fermentation time. It ranged from 0.77 ± 0.03 g/mL (raw sample) to 0.59 ± 0.01 g/mL (LAB consortium from maize fermentation). The lowest reduction was found in LAB consortium from maize fermentation (0.59 ± 0.01 g/mL), followed by LAB consortium from sorghum fermentation (0.61 ± 0.01 g/mL) and then natural fermentation (0.64 ± 0.01 g/mL). The variations in the SC of the sample differ significantly (p<0.05) when compared between unfermented and fermented samples. The result of this study is lower than the report of [30] on fermented Afzelia Africana flour which ranged from 2.50-2.87 g/mL. However, the decrease in the SC during fermentation agreed with the reports of [2] and [6] who reported decrease in SC with increasing fermentation in sorghum, millet, sorghum and pigeon pea respectively. Swelling capacity has been reported to be due to the quantity of amylopectin which are subject to microbial degradation during fermentation [31].
Water holding capacity (WHC) in this study decreased with increasing fermentation time in the range of 2.4 ± 0.03 mL/g to 1.9 ± 0.03 mL/g. There were significant differences (p<0.05) when the 48 h fermented products is compared to the raw sample (0 h). This observation is comparable to the work of [2] who reported decrease in WHC of pigeon pea from 142.0 g/100 g to 113.0 g/100 g after five day fermentation. Similar observation has been reported by [22] on lactic acid bacteria fermented maize flour and [27] on QPM-soybean blend. The values of the present report is higher than 1.12 g/100 g reported by [32] on soybean. Also, the present observation disagrees with the report of [30] who reported increase in WHC of fermented Afzelia Africana . WHC is an indication of the amount of water available for gelatinization and according to [6], low absorption capacity is desirable for making thinner gruels.
In the present study, the oil holding capacity (OHC) of the flour increased significantly (p<0.05) with increasing fermentation time. The highest increase was observed in 48 h sample fermented with LAB consortium from sorghum (9.69 ± 0.03 mL/g), followed by LAB consortium from maize fermented sample (9.63 ± 0.03 mL/g) while the spontaneous fermentation was the least (9.30 ± 0.03 mL/g). This observation agreed with the report of [6] that fermentation increased the OHC in the range of 8.0 to 9.7 for sorghum, millet and maize. Also, Ogodo et al. who reported increase in OHC of maize flour fermented with LAB consortium which is in agreement with the present investigation [22]. However, the result of this study is higher than 1.43 ml/g reported by [1] for soybean. The increase in the OHC of the present study suggests that the flour could be useful in food formulation where an oil holding capacity is considered [6]. Moreover, where optimum oil absorption is desired in food system, the ability of the flours to bind with oil makes it very useful, hence potential for use in foods such as sausages [26].
The least gelation concentration (LGC) concentration in the present study ranged from 3.0% to 6.0%. Adebowale et al. also reported decrease in gelation power with increasing fermentation time in pigeon pea which agreed with the present investigation [2]. The values obtained in the present study are lower than the report of other researchers on similar legumes such as cowpea (16%), soybean flour (10%), pigeon pea flour (4%), and African bread fruit flour (6 to 12%) [32-35]. The variations in the gelation capacities could be attributed to the relative ratios proteins, carbohydrates and lipids that make up the flour which may have a significant impact on the functional properties of the products [2,36].
Emulsion capacity (EC) of soybean flour in the present study increased significantly (p<0.05) with increasing fermentation time. It ranged from 44.33 ± 1.22% (spontaneous fermentation) to 46.83 ± 3.18% (LAB consortium from maize fermentation). The highest increase was observed in LAB consortium from maize fermentation (46.83 ± 3.18%) followed by LAB consortium from sorghum fermentation (45.99 ± 1.18%) while the spontaneous fermentation was the least (44.33 ± 1.22%) after 48 h fermentation. This result is consistent with the reports of [26] who reported EC during fermentation of wheat and rice as 43 and 41% respectively. Also, Ogodo et al. reported increase in EC of maize with increasing fermentation time [22]. However, the present observation did not agree with the report of Igbabul et al. who reported decrease in EC of brown hamburger beans (Mucuna sloanei ) and sweet detar seeds (Detarum microcarpum ) during fermentation [37]. Difference in the EC of the various samples may be related to solubility and hydrophobicity of proteins [26,38].
Conclusion
The functional properties of sorghum flours in the present study after natural fermentation, LAB-consortium from maize and LABconsortium from sorghum fermentations showed improvement. The improvements suggest the potential use of the flour for formulation of infant foods and making of thinner gruels. Hence, the flour can be used to blend foods for improved nutritional qualities. These suggest the possible use LAB-consortium fermentation in improving the nutritional qualities of local staple legume products.
References
- Acuna SPC, Gonzalez JHG, Torres IDA (2012) Physicochemical characteristics and functional properties of vitabosa (Mucuna deeringiana) and soybean (Glycine max). Ciênc Tecnol Aliment 32: 98-105.
- Adebowale OJ, Maliki K (2011) Effect of Fermentation Period on The Chemical Composition and Functional Properties of Pigeon Peas (Cajanus cajan) Seed Flour. Int Food Res J 18: 1329-1333.
- Maidala A, Doma UD, Egbo LM (2013) Effects of Different Processing Methods on the Chemical Composition and Antinutritional Factors of Soybean [Glycine max (L.) Merrill]. Pak J Nutr 12: 1057-1060.
- Vijayakumari K, Siddhuraju P, Janardhanan K (1997) Effect of domestic processing on the levels of certain antinutrients in Prosopis chilensis (Molina) Stunz. Seeds. Food Chem 59: 367-371.
- Kwon DY, Hong SM, Ahn IS, Kim MJ, Yang HJ, et al. (2011) Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effect in vitro. Nutr 27: 244-252.
- Singh A, Yadav N, Sharma S (2012) Effect of fermentation on physicochemical properties & in vitro starch and protein digestibility of selected cereals. Int J Agri Food Sci 2: 66-70.
- Dueñas M, Hernández T, Robredo S, Lamparski G, Estrella I, et al. (2012) Bioactive Phenolic Compounds of Soybean (Glycine max cv. Merit): Modifications by Different Microbiological Fermentations. Polish J Food Nutr Sci 62: 241-250.
- Olanipekun BF, Otunola ET, Oyelade OJ (2015) Effect of Fermentation on Antinutritional Factors and in Vitro Protein Digestibility of Bambara Nut (Voandzeia subterranean L.). Food Sci Qual Manag 39: 98-112.
- Adelakun OE, Duodu KG, Buys E, Olanipekun BF (2013) Potential Use of Soybean Flour (Glycine max) in Food Fortification. INTECH
- Kwon DY, Daily JW, Kim HJ, Park S (2010) Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res 30: 1-13.
- Ramadan MM, Elbandy MA, Fadel M, Ghanem KZ (2014) Biotechnological Production of Volatile and Non-Volatile Antioxidant Compounds From Fermented Soy Bean Meal with Trichoderma sp. Res J Pharm Biol Chem Sci 5: 537-547.
- Randhir R, Vattem D, Shetty K (2004) Solid-state conversion of fava beans by Rhizopus oligosporus for enrichment of phenolic antioxidants and L-DOPA. Innov Food Sci Emerg Technol 5: 235-244.
- Dueñas M, Fernández D, Hernández T, Estrella I, Muñoz R (2005) Bioactive phenolic compounds of cowpeas (Vigna sinensis L.). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J Sci Food Agric 85: 297-304.
- Lin CH, Wei YT, Chou CC (2006) Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol 23: 628-633.
- Wardhani DH, Vázquez JA, Pandiella SS (2009) Mathematical modelling of the development of antioxidant activity in soybeans fermented with Aspergillus oryzae and Aspergillus awamori in the solid state. J Agric Food Chem 57: 540-544.
- Masood MI, Qadir MI, Shirazi JH, Khan IU (2011) Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol 37: 91-98.
- Ogodo AC, Ugbogu OC, Onyeagba RA, Okereke HC (2017) Effect of Lactic Acid Bacteria Consortium Fermentation on the Proximate Composition and in-Vitro Starch/Protein Digestibility of Maize (Zea mays) Flour. Amer J Microbiol Biotechnol 4: 35-43.
- Chelule PK, Mbongwa HP, Carries S, Gqaleni N (2010) Lactic acid fermentation improves the quality of amahewu, a traditional South African maize-based porridge. Food Chem 122: 656-661.
- Huili P, Guangyong Q, Zhongfang T, Zongwei L, Yanping W, et al. (2011) Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst Appl Microbiol 34: 235-241.
- Onyango CA, Ochanda SO, Mwasaru MA, Ochieng JK, Mathooko FM, et al. (2013) Effects of Malting and Fermentation on Anti-Nutrient Reduction and Protein Digestibility of Red Sorghum, White Sorghum and Pearl Millet. J Food Res 2: 41-49.
- Chun J, Kim GM, Lee KW, Choi ID, Kwon GH, et al. (2007) Conversion of isoflavone glucosides to aglycones in soymilk by fermentation with lactic acid bacteria. J Food Sci 72: M39-M44.
- Ogodo AC, Ugbogu OC, Onyeagba RA, Okereke HC, Agwaranze DI (2016) Dynamics of Functional Properties of Maize Flours Fermented With Lactic Acid Bacteria (LAB)-Consortium Isolated From Cereals. FUW Trends in Sci Technol J 1: 134-138.
- Chau CF, Huang YL (2003) Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L. cv. Liucheng. J Agric Food Chem 51: 2615-2618.
- Robertson JA, Monredon FD, Dysseler P, Guillon F, Amado R, et al. (2000) Hydration properties of dietary fibre and resistant starch: a European collaborative study. Lebensm-Wiss u-Technol 33: 72-79.
- Aremu MO, Olaofe O, Akintayo ET, Adeyeye EI (2008) Foaming, Water Absorption, Emulsification and Gelation Properties of Kersting’s Groundnut (Kerstingiella geocarpa) and Bambara Groundnut (Vigna subterranean) Flours as Influenced by Neutral Salts and Their Concentrations. Pak J Nutr 7: 194-201.
- Suresh C, Samsher C (2013) Assessment of functional properties of different flours. Afri J Agric Res 8: 4849-4852.
- Buta MB, Emire SA (2015) Effects of Fermentation on the Nutritional Quality of QPM and Soybean Blends for the Production of Weaning Food. J Food Process Technol 6: 507.
- Lalude LO, Fashakin JB (2006) Development and nutritional assessment of a weaning food from sorghum and oil-seeds. Pak J Nutr 5: 257-260.
- Mesfin W, Shimelis AE (2013) Effect of soybean/cassava flour blend on the proximate composition of Ethiopian traditional bread prepared from quality protein maize. Afri J Food Agric Nutri Develop 13: 7985-8003.
- Igbabul B, Hiikyaa O, Amove J (2014) Effect of Fermentation on the Proximate Composition and Functional Properties of Mahogany Bean (Afzelia africana) Flour. Curr Res Nutr Food Sci 2: 1-7.
- Tester RF, Morrison WR (1990) Swelling and Gelatinization of Cereal Starches. II. Waxy Rice Starches. Cereal Chem 67: 558-563.
- Alfaro MJ, Alvarez I, El Khor S, de Padilla FC (2004) Functional properties of protein products from Caryodendron orinocense (Barinus nut). Arch Latinoam Nutr 54: 223-228.
- Sathe SK, Salunkhe DK (1981) Functional properties of great Northern bean (Phaseolus vulgaris L.) Proteins: Emulsion, foaming, viscosity and gelation properties. J Food Sci 46: 71-81.
- Onimawo IA, Momoh AH, Usman A (1998) Proximate composition and Functional Properties of four cultivars of Bambara groundnut. Plant Foods Hum Nutr 53: 153-158.
- Fasasi OS, Eleyinmi AF, Oyarekua MA (2007) Effect of some traditional processing operation on the functional properties of African breadfruit seed (Treculia africana) flour. LWT-Food Sci Technol 40: 513-519.
- Sathe SK, Deshpande SS, Salunkhe DK (1982) Functional properties of Lupin seed (Lupinus mutabilis) protein and protein concentrates. J Food Sci 47: 491-497.
- Igbabul BD, Idikwu HO, Inyang CU (2012) Effect of fermentation on some functional properties of Mucuna sloanei and Detarium microcapum. J Food Technol 10: 83-86.
- Kaushal P, Kumar V, Sharma HK (2012) Comparative study of physico-chemical, functional, anti-nutritional and pasting properties of taro (Colocasia esculenta), rice (Oryza sativa), pigeonpea (Cajanus cajan) flour and their blends. LWT-Food Sci Technol 48: 59-68.
Copyright: © 2017 Ogodo AC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.