Journal of Hematology & Thromboembolic Diseases
Open Access
ISSN: 2329-8790
ISSN: 2329-8790
Mini Review - (2024)Volume 12, Issue 6
The old veto effect may be moving from its status as an obscure immune inhibitory phenomenon to an important role on specific immune suppression. In today's clinical practice, broad suppression of the immune system is employed to avoid transplant rejection and mitigate auto-aggressive immune responses. Though highly effective, this approach impairs immune protection against infectious challenges. Therapeutic approaches are being sought that specifically inhibit sections of the immune system without affecting beneficial immune functions. One such tactic entails the classical veto-effect that employs donor-derived CD8+ T cells to inhibit cellular immune responses. Indeed, this kind of veto has already found applications as the underpinning of a more broadly applicable CAR-T cell therapy and of a more spec0069fic immune suppression for haploidentical Hematopoietic Stem Cell (HSC) transplantations. To broaden and simplify the use of veto, engineering strategies are discussed that affix its immune inhibitory function to cells of different tissues. They are based on the transfer of the CD8 α-chain to the surface of different cell populations. We predict that engineered veto will simplify specific immune suppression and broadening its application to organ transplantations and possibly the treatment of autoimmune diseases. It may represent an avenue to induce immunological tolerance.
Immune system; CAR-T cell therapy; hematopoietic stem cell transplantations
The word veto is derived from the Latin verb vertare and literally translated means I forbid. Veto was first described as an obscure immune inhibitory phenomenon that was induced by the injection of CD8+ T cells into mice [1]. It was subsequently shown that this classical veto removed T cells from the peripheral repertoire in a highly specific and effective manner. T cells are guided through their development in the thymus and their fate in the periphery by positive and negative interactions. Immature T cells undergo both expansion and deletion events [2]. Bone marrow-derived precursors migrate to the thymus, where T cell differentiation begins with the rearrangement of the T Cell Antigen Receptor (TCR) variable genes. The assembled TCRs are clonally expressed on the surface of thymocytes. During positive selection the immature T cells are screened for their ability to recognize antigens in a given Major Histocompatibility Complex (MHC) environment. Only the successful cells are allowed to proceed. Strongly auto-reactive T cells are removed in another thymic process. During negative selection, they are either removed from the repertoire or inactivated when they react with self at high affinities. Having completed their development, T cells in the periphery are induced when they exposed to their cognate antigen presented by professional Antigen Presenting Cells (APC). T cells are fully activated by the engagement of their TCR in concert with a costimulatory molecule [3]. They are inhibited when their specific antigen is not presented on professional APCs. Their activity can also be inhibited by regulatory T cells [4]. It is not known whether veto inhibition represents a physiological mechanism to preserve peripheral tolerance.
The mechanistical underpinning of classical veto differs from other peripheral processes. Veto was first seen when animals were injected with lymphocyte populations enriched for Cytotoxic T Lymphocytes (CTL) [1]. It was found that CTL precursors that had been exposed to their antigen on CD8+ T cells were removed from the peripheral T cell repertoire. Veto was shown to inhibit the induction of T cells with specificities for allogeneic MHC, as well as minor and haptenated histocompatibility antigens. In its classical form, the veto-ing T cell is passive. It does not have to recognize the inhibited cell, but rather must be recognized to delete the responsive T cells. Thus, the specificity of the veto-ing CD8+ T cell is not crucial for this form of specific immune inhibition. Although it was shown that a retrograde killing of the CD8+ T cells enhanced veto inhibition, it was not mandatory. It was found that also nonlytic CD8+ Bone-Marrow (BM) cells elicited veto inhibition [5]. Further mechanistical studies demonstrated that a trigger of the veto inhibitory function rested in the CD8 α-chain. Deleting the expression of the CD8 α-chain removed the ability of cells to veto [6,7]. Cells gained this function after they had acquired the CD8 α-chain on their surfaces. Having established the central role of the CD8 α-chain, the most straightforward explanation of classical veto employs a co-triggering paradigm (Figure 1).
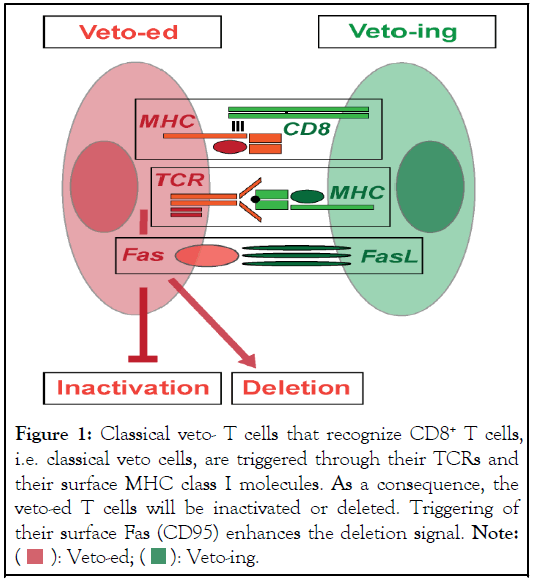
Figure 1: Classical veto- T cells that recognize CD8+ T cells,
i.e. classical veto cells, are triggered through their TCRs and
their surface MHC class I molecules. As a consequence, the
veto-ed T cells will be inactivated or deleted. Triggering of
their surface Fas (CD95) enhances the deletion signal. Note: 
In this model, T cells are veto-ed when they receive signals through the TCR complex concurrently with triggers through the α3 domain of their surface MHC class I molecules. Experiments indeed demonstrated that blocking the CD8 binding to the α3 domain of MHC class I molecules on the responding T cells prevented veto inhibition. Receiving a death signal through MHC class I is not implausible. It was found that certain antibodies that bind to the α3 domain of MHC class I molecules induced cell death. This co-triggering theory also explains how both CD4+ and CD8-independent T cells are inhibited by classical veto. Besides a silencing of the responding T cells, veto can also result in their deletion. Co-expression of CD95L with the CD8 α-chain strongly enhances veto-like inhibition that can lead to the death of the triggered cell (Figure 1)[8]. This lethal veto trigger might also be provided by a release of cytotoxic granules. Even though expression of CD95L on some Hematopoietic Stem Cells (HSC) or on other tissues can lead by itself to the apoptosis of activated T cells, this version of inhibition is distinct from classical veto as it does not depend on the engagement of the TCR and is therefore non-antigen specific.
The redirecting of T cell activities has delivered major breakthroughs in the therapy of malignancies. The powers of CD8+ CTLs in eliminating unwanted cells were harnessed when the unrestricted recognition of an antibody was attached to TCR complexes. In one system, bispecific antibody constructs were developed, in which one binding site was linked to components of the anti-TCR complex and the other one was directed against a chosen tumor antigen [9,10]. With this approach, a patient’s own T cells can be swiftly recruited to decimate malignant cells. This approach has been successfully deployed in the clinic to the treatment of certain lymphomas [11]. In another system, Chimeric Antigen Receptors (CAR) were assembled that grafted antibody variable regions onto TCR signaling proteins. CAR-engineering of autologous T cells requires an efficient and repeated harvesting of large numbers of cells. This is followed by time-consuming manufacturing processes to transfer the CAR T cells before they can be reinfused to the patient [12]. A simplification of this therapy would be achieved if off-the-shelf pre-manufactured CAR T cells could be used. Indeed, classical veto might facilitate the transfer of allogeneic CTLs. Here, CAR-engineered CD8+ T cells from an unrelated doner would provide the therapeutic moiety. Their intrinsic veto activities would negate the attacks by the recipient’s T cells [13]. Clinical trials have demonstrated the feasibility of this idea. Graft-versus-Host Disease (GvHD) phenomena were observed in some patients. They were most likely caused by the presence of the original TCRs on the CAR T cells. Deleting these second specificities should resolve the GvHD effects.
The rejection of HSC grafts was another issue that was addressed by classical veto. It was reported that in the animal the rejection of mismatched HSCs was mitigated when donorderived CD8+ veto cells were added. These findings were confirmed in clinical trials, in which the addition of donorderived T cells lessened the rejection of the haploidentical HSC grafts [14].
Engineering veto immune suppression
Classical veto depends on the activity of cells that, under physiological conditions, express the CD8 α-chain. Yet, CD8 is only expressed on few cells, such as on a subgroup of peripheral T cells, on minor populations of the BM, and on some rare dendritic cells. Veto is mediated by TCR recognition in conjunction with triggering through the α 3 domain of the MHC class I molecules. An infusion of CD8-bearing cells, such as CTLs, only inhibits T cells that are responsive to antigens expressed on veto-ing cells. T cells reactive with antigens selectively expressed on non-CD8 bearing tissues syngeneic to the inhibitory cells remain unscathed. Although the major thrust of transplant rejection is caused by T cells that recognize foreign MHC molecules, T cells that specifically recognize tissue-antigens presented on allogenic MHC molecules participate in graft rejection. The situation is different for autoimmune phenomena. Here, tissue-specific T cells are the principal drivers of the diseases. To use veto to limit auto-aggression and to mitigate transplant rejection, its immune suppression must be expanded to tissue-specific T cells. Two engineering technologies were investigated by us to link veto to different tissues (Figure 2).
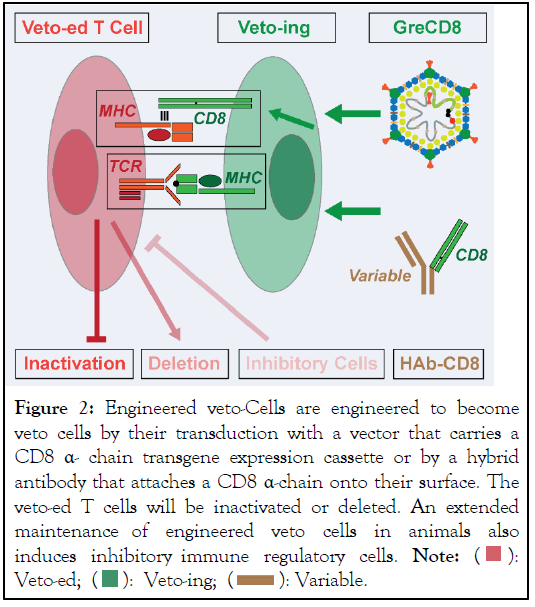
Figure 2: Engineered veto-Cells are engineered to become
veto cells by their transduction with a vector that carries a
CD8 α- chain transgene expression cassette or by a hybrid
antibody that attaches a CD8 α-chain onto their surface. The
veto-ed T cells will be inactivated or deleted. An extended
maintenance of engineered veto cells in animals also
induces inhibitory immune regulatory cells. Note: 
We constructed Hybrid Antibodies (HAb) that linked an antibody variable region specific for an MHC class I molecule to the CD8 α-chain [15,16]. We were able to demonstrate that these HAbs effectively transferred veto inhibition to different cell populations. Once the feasibility of veto engineering had been established, we decided to rigorously probe its effectiveness in vivo. HAbs are not maintained long-term on cells. Nevertheless, their surface half-lives might suffice to resolve an autoimmune attack. As the immune system takes several days to fully activate allo-reactive T cells, it is unlikely that HAbs can effectively evade transplantation rejection. Having investigated different gene transfer systems, we settled on Adenoviral (Ad) vectors that efficiently transduced both proliferating and resting cells of most phenotypes and had the ability to promptly induce transgene expression. In contrast to retroviral and adeno-associated virus DNA, Ad genomes rarely integrate into the host genome. Yet, as they are maintained as episomes, they are nevertheless preserved in cells for extended periods of time. We constructed Ad vectors that carried either the mouse or human CD8 α-Chain as Transgenes (AdCD8) [17]. Once we had established in vitro that different cells transduced with either vector specifically inhibited the induction of allogeneic CTLs, we moved to a mouse transplantation model. We were able to demonstrate that AdCD8 transduced pancreatic islets were permanently (i.e. lifelong) protected from rejection in fully allogeneic hosts (Table 1).
| Groups | Set-up | Donor | Recipient | Pancreatic islets | Graft survival | ||
|---|---|---|---|---|---|---|---|
| Number | Islet treatment | [%] | |||||
| Average | SD | ||||||
| Control#1 | Syngeneic | C57Bl/6 | C57Bl/6 | 789 | 231 | none | 100 |
| Control#2 | Allogeneic | C57Bl/6 | Balb/c | 788 | 104 | none | 0 |
| 450 | 10 | none | 0 | ||||
| Control#3 | Allogeneic | C57Bl/6 | Balb/c | 838 | 46 | Ad(empty) | 0 |
| Veto#1 | Allogeneic | C57Bl/6 | Balb/c | 791 | 85 | AdCD8 | 83 |
| Veto#2 | Allogeneic | C57Bl/6 | Balb/c | 450 | 10 | AdCD8 | 91 |
Table 1: Pancreatic islet transplantation (pancreatic islets harvested from C57Bl/6 mice were treated as listed in the Table. They were transplanted under the kidney capsule of Balb/c mice suffering from chemically induced diabetes mellitus).
No supportive immune suppression was required. Having induced tissue-specific immune inhibition, if not tolerance, it was not unexpected that the rejection of donor-type skin grafts was not fully prevented. This might have been due to the small number of transferred CD8-bearing islet cells as well as the presence of tissue-specific, i.e. skin specific, allogeneic T cells. If skin-patches were modified themselves to express the CD8 α- chain, their rejection was prevented (unpublished observation).
Tissue survival might have ultimately been supported by another immune inhibitory effect. We observed that the long term presence of the islet grafts expanded the activity of regulatory T cells. Liver transplantation has been used for patients with liverbased metabolic disorder and end-stage liver failures. As alternative treatment, hepatocyte transplantation has been investigated. This treatment’s theoretical advantages are severalfold. A less invasive operation is needed. The hepatocytes can be genetically manipulated ex vivo and can be cryopreserved. Cells from a single donor can be provided to several recipients. More than 150 clinical cases of hepatocyte transplantation have been reported. The different trials have established immune rejection as a crucial hurdle to its success. A recent hepatocyte transplantation of a patient with Crigler-Najjar suggested that CD8+ T cells were the primary driver of graft rejection. This observation could point to veto as the venue to facilitate hepatocyte acceptance. We therefore tested in mice whether hepatocytes transduced with AdCD8 would be accepted in allogeneic hosts. The cells were harvested from Balb/c mice, transduced with AdCD8, and then injected into the spleens of C57Bl/6 mice. Similarly to allogeneic pancreatic islets, the hepatocytes survived this highly immune active environment. Since these studies were performed, we have optimized our Ad vector system and have moved to Ad vectors, whose genomes are fully deleted of all endogenous Ad genes [18-20]. These new vectors show little impact on the physiology of infected cells. Therefore, veto vectors based on this 4th generation Ad vector technology provide a unique opportunity to advance engineered veto into the clinic.
Classical veto has already found applications as the underpinning of a more broadly applicable CAR-T cell therapy and of more specific immune suppression for haploidentical HSC transplantations. Engineered veto will simplify and broaden its use to different cell and organ transplantations and possibly the treatment of certain autoimmune diseases. It may represent an avenue to induce immunological tolerance.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Staerz UD, Cull JW, Qi Y (2024) Veto-Engineering Immune Tolerance. J Hematol Thrombo Dis. 12:609.
Received: 14-Jun-2024, Manuscript No. JHTD-24-32036 (PQ); Editor assigned: 17-Jun-2024, Pre QC No. JHTD-24-32036 (PQ); Reviewed: 01-Jul-2024, QC No. JHTD-24-32036 (PQ); Revised: 08-Jul-2024, Manuscript No. JHTD-24-32036 (PQ); Published: 15-Jul-2024 , DOI: 10.35248/2329-8790.24.12.609
Copyright: © 2024 Staerz UD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.