
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)Volume 10, Issue 4
Aim: The aim of this study was to evaluate the technical feasibility and limitations of video-assisted mediastinoscopic
lymphadenectomy (VAMLA) followed by video-assisted thoracoscopic surgery (VATS) lobectomy and video-assisted
thoracoscopic surgery (VATS) lobectomy alone in treating patients with non-small cell lung cancer.
VATS lobectomy alone or following VAMLA is feasible and can be done with an acceptable safety profile under the
hands of specialized, highly trained and cooperating team working in a high volume center treating patients with lung
cancer. VAMLA followed by VATS lobectomy allowed the excision of more lymph nodes compared to the VATS
approach alone, suggesting that VAMLA is a good adjuvant to VATS lobectomy for complete radical mediastinal
lymphadenectomy for the surgical cure of non-small cell lung cancer patients.
Over the last decade we witnessed significant change of practice in many thoracic units within the hands of a new
generation of young minimally invasive thoracic Surgeons. The goal of our research was to evaluate the technical
feasibility and limitations of video-assisted mediastinoscopic lymphadenectomy (VAMLA) followed by video-assisted
thoracoscopic surgery (VATS) lobectomy and video-assisted thoracoscopic surgery (VATS) lobectomy alone in treating
patients with non-small cell lung cancer. A prospective study from September 2015 to September 2016 involving 22
non-small cell lung cancer patients admitted to the Department of Thoracic Surgery of the Referral Oncologic
Center of Basilicata (IRCCS-CROB), Italy, was done. Six patients underwent a combination of subsequent VAMLA
and VATS lobectomy (Group A), whereas sixteen patients underwent lobectomy and mediastinal lymphadenectomy
using thoracoscopy only (Group B). Comparison between the two studied groups was done regarding the baseline
characteristics, operative profiles and complications. Males were more than females (17 patients vs. 5 patients)
respectively. The most common tumour was T1 (18 patients). And, the most common encountered tumour was
adenocarcinoma (17 Patients). Our results highlighted that the lobectomy operative time was shorter in (Group A),
(117 minutes) compared to (Group B), (157.5 minutes). The total number of mediastinal lymph nodes excised in
(Group A), (18 lymph nodes) was more than (Group B), (12.5 lymph nodes). VATS lobectomy alone or following
VAMLA is feasible and can be done safely under the hands of specialized, highly trained and cooperating team
working in a high volume center treating lung cancer patients.
Video-assisted Mediastinoscopic Lymphadenectomy (VAMLA); Video-assisted Thoracoscopic Surgery (VATS); Lobectomy and Lung Cancer
CT: Computed Tomography; Ctnm: Clinical Stage; Ebus: Endobronchial Ultrasound; Ests: European Society of Thoracic Surgery; Eu: Endoscopic Ultrasound; Fdg: Fluorodeoxyglucose; Hd: High Definition; Iaslc: International Association for the study of Lung Cancer; Irccs-Crob: Referral Oncologic Center of Basilicata, Italy; Nscc: Non–Small Cell Carcinoma; Pet: Positron Emission Tomography; Ptnm: Pathological Stage; R0: No Cancer Cells Seen Microscopically at the Primary Tumour Site; R1: Cancer Cells Present Microscopically at the Primary Tumour Site; Sclc: Small Cell Lung Carcinoma; Vamla: Video Assisted Mediastinoscopic Lymphadenectomy; Vats: Video- AssistedThoracoscopic Surgery
Thirty years after Carlens introduced mediastinoscopy in 1959; added video support to the instrument. In 1992, Linder et al. developed the spreadable video-mediastinoscope. This introduced scope allowed for bimanual dissection, and its invention was the advent of mediastinoscopic surgery, in contrast to purely diagnostic mediastinoscopy, that had been performed until then. One of the new operations becoming apparent from mediastinoscopic surgery was video assisted mediastinoscopic lymphadenectomy (VAMLA), which Witte et al. [1] developed and reported on the first time in 2002. The well-established VAMLA has been closely connected to the emergence of multimodal treatment of lung cancer and thoracoscopic lobectomy.
Despite the fact that the first VATS lobectomy was performed in Italy in October 1991 by Roviaro, the uptake of procedure has been relatively slow in Italy. Between then and December 2012 a total of 1,366 VATS lobectomies have been carried out in Italy. Twelve centers performed over 30 cases and only three centers performed over 100 cases [2].
Despite significant seeding of such procedure in several thoracic units globally, the uptake and the learning curve was slow and frustrating. The majority of surgeons considered it complicated and unsafe being in doubt about its oncological validity [3].
In 2011, the highest VATS resection rate across Europe was seen in Denmark, with 55% of lobectomies was performed via a VATS approach. These cases were done in four specialized units operating between 100 and 325 lung cancer surgeries per year. The team in Copenhagen has the largest experience in Europe with more than 1,500 cases done and 80% of the procedures being carried out via VATS. Their experience is written in the medical literature [4].
Over the last ten years we witnessed significant change of practice in many thoracic units within the hands of with a new generation of young minimally invasive thoracic Surgeons. Additionally the technique has been refined, standardized and proved its validity and superiority in lung cancer treatment [3].
Lung cancer is the leading cause of cancer death in both men and women. Clinical staging plays an important role in predicting survivor as well as directing treatment options in lung cancer patients.
Guidelines are constantly being reviewed and revised as more data becomes available to provide the most accurate prognostic markers, hence helping in the clinical detection and accurate staging of lung cancer.
Since its introduction in the 1970s, the TNM staging has undergone many revisions with the latest, 8th edition, being effective internationally from 2018 [5].
Staging of NSCLC involves multiple diagnostic modalities; guidelines recommend use of combined positron emission tomography (PET) and computed tomography (CT) if available, and otherwise a CT scan alone [6].
Mediastinal nodes with significant fluorodeoxyglucose (FDG) uptake or with a small axis diameter greater than 1 cm on the scan are considered suspicious. So, minimally invasive techniques are recommended to obtain a tissue diagnosis of these nodes. Endoscopic ultrasound (EUS) or endobronchial ultrasounds (EBUS) are the techniques used if available under the hands of an experienced operator. If these prove to be negative or if not available, mediastinoscopy is recommended to be the next step.
In patients carrying a risk of mediastinal lymph node metastasis, with a primary tumor >3 cm, a central tumor or a tumor with suspicious N1disease, pathological staging of the mediastinum is also recommended, starting with EUS/EBUS. If results are negative but suspicion is high a mediastinoscopy is recommended as well [7].
Video-assisted thoracoscopic surgery (VATS) is a minimally invasive surgical modality being used for both diagnostic and therapeutic purposes for lung cancer patients. It offers low perioperative morbidity and mortality as well as decreased pain and hospitalization [8].
Recurrence rates and 5-year and long-term overall survival appear similar to those with traditional open thoracotomies. This approach is also better tolerated in older populations. Finally, patients treated with VATS appear to have fewer delays and dose reductions in adjuvant chemotherapy related to the enhanced recovery following the minimally invasive procedure. Practice guidelines suggest that VATS is feasible as long as adequate resection is possible [9].
The perioperative mortality rate is 6% for pneumonectomy, 3% for lobectomy, and 1% for segmentectomy. These rates reflect improvements in anaesthesia and surgical techniques observed nowadays [10].
Between September 2015 and September 2016 twenty-two non-small cell lung cancer patients admitted to the Department of Thoracic Surgery of the Referral Oncologic Center of Basilicata (IRCCS-CROB), Italy, were studied prospectively. After admission, all patients were subjected to complete history taking regarding; personal data, past history, symptoms, smoking status and duration. Then, patients were subjected to clinical assessment by recording vital signs, doing chest examination and evaluation of all body systems. Routine laboratory investigations were done including complete blood picture, fasting blood glucose, coagulation profile, renal function tests and liver function tests. Arterial blood gases analysis, Spirometry, resting 12 leads ECG and Trans-thoracic Echocardiography were done to evaluate the preoperative fitness of the patients followed by anaesthesiology consultation. Flexible bronchosopic examination for all patients was done. However, endoscopic ultrasound (EUS) and endobronchical ultrasound (EBUS) were not available at our center. According to the ESTS guideline 2007, a recent PET and CT or PET-CT was done for each patient. Patients with cN0 disease and with peripheral tumors less than 3 cm were subjected directly to VATS Lobectomy (Group B), (Figure 1). While, Patients with cN1, cN2 disease, central tumors, tumours>3 cm or nodules showing high FDG uptake value; were subjected to VAMLA followed by VATS Lobectomy (Group A) unless VAMLA precludes further surgery by showing an evidence of multiple stations N2 disease or any N3 disease.
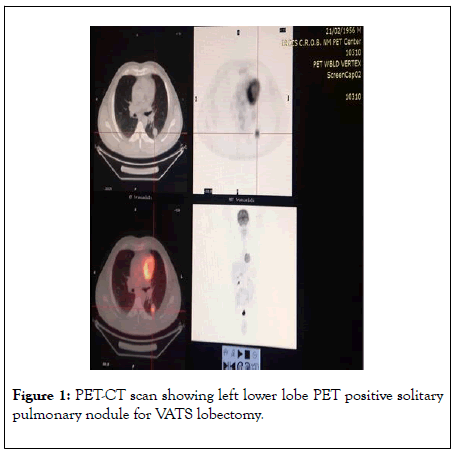
Figure 1: PET-CT scan showing left lower lobe PET positive solitary pulmonary nodule for VATS lobectomy.
Surgical technique for VAMLA
The preparation of the pretracheal space and the introduction of the closed video-mediastinoscope are the same as those employed for standard mediastinoscopy. The patient is intubated with a single-lumen endotracheal tube under general anaesthesia and placed in a supine position with a neck hyperextended. After the pre-tracheal and para-tracheal fasciae are opened and blunt dissection is performed, the two-bladed video mediastinoscope is inserted and advanced into the pretracheal space. After the inferior valve of the video mediastinoscope is opened to create the wide operative field enough for bimanual maneuver, the node stations including station 2 on the right (2R) and left (2L), station 4 on the right (4R) and left (4L), and station (7) were explored. Whenever possible, we usually performed en bloc resection along with neighbouring fatty tissue for all accessible lymph nodes in the right para-tracheal (Figure 2) and subcarinal compartments. For the excision of the leftsided node stations, careful dissection was made not to injure the adjacent left recurrent laryngeal nerve.
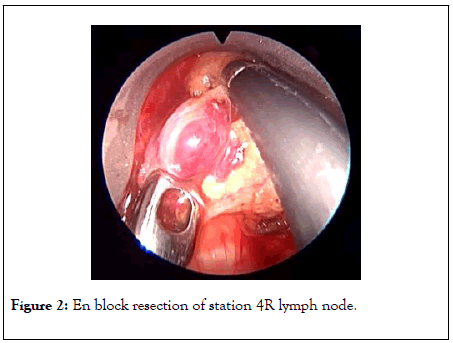
Figure 2: En block resection of station 4R lymph node.
When dissecting the subcarinal region, the specimen consisting of lymph nodes and the mediastinal fat tissue is first separated from the medial parts of both main bronchi and from the edge of the bifurcation. Then by distal dissection of about 3 cm the complete subcarinal region is explored. The main bronchi can be exposed more easily on the right than on the left side ventrally towards the upper lobe origin. Here, the lymph nodes are often attached to the pulmonary artery or its superior trunk and require careful preparation. On the left side, the upper pulmonary vein becomes visible sometimes caudal to the pulmonary artery. Finally, the esophagus is exposed between the two main bronchi.
Video-assisted thoracoscopic surgery (VATS) Lobectomy
We used the standardized three-port anterior approach used by The team in Copenhagenn [7], with sequential division of the hilar structures, proper lymph node handling, no rib spreading and vision relying on the monitor only. This allowed us to perform VATS lobectomies in the majority of the cases even if there were significant difficulties.
Indications for VATS lobectomy
Video-Assisted thoracoscopic surgery (VATS) lobectomy was performed for selected peripherally located T1 or T2 tumours and usually reserved for patient where complications are not expected.
Contraindications: T3 or T4 tumours. Tumours larger than 6 cm.Tumours visible in the bronchus by bronchoscopy within 2 cm of the origin of the lobe to be resected and where a possible sleeve resection might be needed.
Centrally placed tumours in the hilum and adherent to vessels.
Operating room set-up and basic surgical principles
A standard set-up is with one monitor placed on each side of the table in front of the surgeons and the scrub nurse. Other screens in the room allow other persons in the theatre to follow the surgery. We have dedicated VATS theatre together with Olympus and this theatre is only used for thoracic procedures.
The basic principle is that the theatre is symmetrical so it is suitable for both right and left sided procedures. The light setting is a dynamic and coloured lighting that enhances the surgical ergonometry.
All VATS lobectomies were performed with a 10 mm, 30 degree angled HD video-thoracoscope. The 30-degree angulation allows a superior view within the chest cavity. In a 10 mm camera, the power of the light source is stronger than the light source in the existing 5 mm cameras, and is not easily flooded by even a minor bleeding. The surgeon and the assistant are positioned on the anterior (abdominal) side of the patient and with the surgeon cranially. The scrub nurse is opposite to the assistant and follows the operation on a separate screen and still positioned face to face with the surgeon.
Initially, a 5 cm anterior utility incision was made without any tissue retractor or rib spreading. The wound is protected by a plastic soft tissue retractor kept in place by a ring in the chest cavity and one outside the skin (Alexis Retractor) (Figure 3). This incision is later used for specimen retrieval, and is positioned between the breast and the lower angel of the scapula in the fourth intercostal space just anterior to the latissimusdorsi muscle. In case of a conversion to open procedure, this incision can be easily expanded to a 10 to 15 cm thoracotomy within a few minutes.
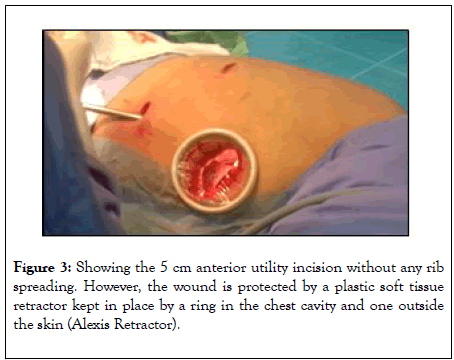
Figure 3: Showing the 5 cm anterior utility incision without any rib spreading. However, the wound is protected by a plastic soft tissue retractor kept in place by a ring in the chest cavity and one outside the skin (Alexis Retractor).
The pleural cavity is evaluated with the camera through this incision looking for unexpected pathology, adhesions, and the level of the diaphragm. A low anterior 1 cm camera-port is positioned at the level of the top of the diaphragm and anterior to the level of the hilum and the phrenic nerve. The final 1.5 cm incision is positioned at the same level but more posterior in a straight line down from the scapula and anterior to the latissimusdorsi muscle. This results in a triangle with two approximately 10 cm limbs and the camera positioned at the apex, with a working channel on each side, which makes the procedure more easy and natural to the surgeon. The camera is in the lower anterior corner of the chest cavity with a good overview and it is usually not necessary to change camera port at any point of the procedure.
To palpate, free and prepare the structures, we used an array of a peanut or a sponge stick, an electrocautery blade hook controlled with a normal surgical handhold. The tip of the hook can be used to lift and divide the tissue. To present vessels and other structures to be divided we use vicryl 3/0, as sling for materials present a risk of tearing, especially the fragile arteries to upper lobes. We did not place clamps on the vessels before stapling but two vessel clamps are ready on the table in case of an emergency bleeding and furthermore a set up for open surgery is present in the theatre.
The vessels, the fissures and the bronchus are divided sequentially, with appropriate endostaplers. For the vessels and thin parenchyma we used a tan Tri-stapler (Covidien, USA) and for poorly defined fissures and the bronchus, we transect with a purple Tri-stapler. Any specimen with suspicion of malignancy is removed with an endobag [11].
Energy-based devices can also be used and we had some experience with an electro-thermal bipolar tissue sealing system (Ligasure) and find it useful to transect minor pulmonary arteries up to the size of 3-4 mm and they are very useful for lymphadenectomy where it facilitates an “en bloc” dissection.
At the end, one intercostal drain is placed in through the camera port (Figure 4). After surgery, the patient is transferred to an intermediate ward and next day to the normal ward. The patients were mobilized on the day of surgery and lung physiotherapy is provided for training. The tube was removed when there is no air-leakage and less than 200 cc of fluid in 24 hours. Patients were usually discharged one day after tube removal and seen ten days later in the outpatient clinic.
Figure 4: Showing one intercostal drain placed in through the anterior inferior incision.
The total number of patients is 22, 6 patients did VAMLA followed by VATS lobectomy (Group A), whereas 16 underwent lobectomy and mediastinal lymphadenectomy using thoracoscopy only (Group B).In the Group A (n=6), there were 6 (100%) males with a mean age of 69.17 ± 5.95 years, whereas there were 11 (68.8%) males with a mean age of 68.19 ± 8.17 years in Group B.
Clinical (cT1) tumour was the most common stage in both groups, seen in 13 patients (81.3%) in Group B vs. 5 patients (83.3%) in Group A.
In Group B, 3 patients (18.8%) had cT2 tumours compared to 1 patient (16.7%) in Group A. All patients (100%) in Group B were cN0 disease. In Group A, 6 patients (100%) had cN2 disease vs. no patients with cN2 disease in Group B.
According to the location of the tumour, in Group B, the right upper lobe was the most common site of the tumour (37.5%) vs. (16.7%) in Group A. Adenocarcinoma was the most common histopathological type seen Group B (87.5%) vs. (50%) seen in Group A.
The mean operative time for VAMLA alone was 42.0 ± 16.4 minutes. The median lobectomy operative time for Group B was 157.50 minutes vs. 117.0 minutes for Group A .The median total number of lymph nodes removed (18.0) was significantly higher in Group A compared to (12.50) in Group B (Table 1).
| Group B | Group A | Test of significant | p | |
|---|---|---|---|---|
| (n=16) | (n=6) | |||
| Lobectomy Operative time (minutes) | ||||
| Min. – Max. | 120.0 – 300.0 | 110.0 – 162.0 | t= | 0.017* |
| Mean ± SD. | 166.25 ± 43.95 | 126.67 ± 20.84 | 0.084 | |
| Median | 157.5 | 117 | ||
| Number of nodes excised | ||||
| Min. – Max. | 2.0 – 25.0 | 5.0 – 31.0 | U= | 0.023* |
| Mean ± SD. | 13.44 ± 7.66 | 17.83 ± 10.67 | 15.50* | |
| Median | 12.5 | 18 | ||
t, p: t and p values for Student t-test for comparing between the two groups.
U, p: U and p values for Mann Whitney test for comparing between the two groups.
*: Statistically significant at p ≤ 0.05.
Table 1: Comparison between the two studied groups according to the lobectomy operating time and the total number of lymph nodes excised.
In both groups none of the patients required admission to the intensive care unit. Also, there was no difference in the length of hospital stay between the two groups. The median was 7 days for Group B and 6 days for Group A. The complications seen in were a single case of prolonged air leak, a single case of anaemia in Group B and a single case of atrial fibrillation in Group A.
In Group B, 5 patients (31.3%) were converted to open surgery vs. 2 patients (33.3%) in Group A. Group A, achieved R0 resection in all patients (100%) vs. 14 patients (87.5%) in group B. There was no in-hospital mortality in both groups.
In our results, we showed that males were more than females in both groups. However, Witteet al. [12] demonstrated the same result only in the VATS and VAMLA group, while, in the VATS only group females were more than males.
Also, our studied mean age in both groups was around 68 years. Approximately the same mean age seen in both groups studied by Witte et al. [12].
Non-small cell lung cancer occurs mainly in persons aged 50-70 years. The probability of developing lung cancer remains very low until age 39 years in both sexes. The risk of developing lung cancer remains higher among men in all age groups after age 40 years [13].
Regarding the tumour histology, all our patients had either adenocarcinoma or squamous cell carcinoma. Compared again to the study done by Witte et al. [12] the latter study identified adenocarcinoma, squamous cell carcinoma, small-cell lung cancer, carcinoid, Bronchoalveolar carcinoma and solitary lung metastasis. However, adenocarcinoma was the most common tumour type seen in both studies.
The histopathology of lung cancer is changing. The incidence of squamous cell carcinoma is decreasing, accompanied by the increase in the incidence of adenocarcinoma [14].
Accurate mediastinal staging of resectable lung cancer is a clinically important in the prediction of postoperative prognosis and the guidance for the following step in adjuvant therapy [15].
Among the three components of TNM staging system, the nodal stage is the most difficult to determine accurately due to the limited specificity and sensitivity of different imaging modalities compared to the conventional Carlens mediastinoscopy [16].
However, the limited visualization of the mediastinal structures by the conventional Carlens cervical mediastinoscopy has forbidden the complete resection of the mediastinal lymph nodes [16].
In our study, in Group A, all the patients had cN2 disease and they underwent a VAMLA procedure followed by a VATS lobectomy. Furthermore, the mean total number of mediastinal lymph nodes excised in Group A (17.83 ± 10.67) was significantly more than in Group B (13.44 ± 7.66). This finding coincide with the study done by Kim et al. [17], that showed more extensive lymph node dissection for the patients in the VATS and VAMLA group (total number of removed lymph nodes, 29.7 ± 10.8 vs. 23.0 ± 8.6, p<0.001).
We do agree with the conclusions published by Leschber et al. denoting that mediastinal lymph node staging is improved and is more accurate by VAMLA, where a systematic lymphadenectomy is performed and the total number of lymph nodes removed is increased [18].
The telescope and the two bladed expandable speculum used in VAMLA allowed the operating surgeon to perform a more extensive mediastinal lymph node dissection, which helped the surgeon to do a bimanual maneuver and gave him a magnified wider operative field [19].
In addition, we demonstrated that the mean operative time for VATS lobectomy is significantly shorter in group A (126.67 ± 20.89 minutes) compared to the VATS lobectomy time in group B (166.25 ± 43.95 minutes). And the shortened time is explained by the time saved for mediastinal lymph nodes dissection with VAMLA. Similar findings were documented by Kim et al. [17], (116.8 ± 39.8 vs. 159 ± 44.0 minutes, p<0.001).
Thus, it is logic to say that regarding N2 disease, a VAMLA procedure followed by VATS lobectomy can accurately evaluate lymph node metastasis and can be a fundamental component of lung cancer staging. In addition, it significantly affects decisions and the multidisciplinary treatment approach in patients with non-small cell lung cancer.
VAMLA is of great value to identify N2 disease in patients eligible for neoadjuvant therapy. Moreover, more importantly prior to video-assisted thoracoscopic lobectomy, lymph node metastasis can be excluded with high sensitivity and make the thoracoscopic procedure much easier [20].
It was reported in the previous medical literature that the sensitivity of VAMLA is higher than a conventional mediastinoscopy. And this lies behind the fact that the sensitivity of mediastinoscopy is affected by the amount and number of removed lymph nodes and lymph node stations [19].
As far as mediastinal lymph nodes staging is concerned, VAMLA is known to be as accurate as open mediastinal lymph nodes dissection. VAMLA is not only used diagnostically for staging the mediastinum in lung cancer, but also it is important therapeutic tool for complete mediastinal lymph nodes dissection, and it is gaining wider acceptance as a complement to VATS lobectomy for lung cancer treatment.
In addition, VAMLA presents the basis for video-assisted thoracic surgery (VATS) lobectomy because complete resection of the mediastinal lymph nodes can be achieved [18].
Moreover, accurate evaluation of lymph node metastasis is a fundamental component of cancer staging, and it significantly affects decisions and multidisciplinary treatment approach in patients with non-small cell lung cancer [21].
In the 8th edition of the TNM Classification of Malignant Tumours, the 4 pathological categories (N0, N1, N2, and N3) remained the same as first proposed in the previous 7th edition, which is exclusively determined by the location of involved nodes [22].
Multiple numbers of studies have reported that patients with the same N status may have different prognosis. Because of the intrinsic heterogeneity of N2 disease, treatment strategy including the best combination and sequence for the multimodality therapy for N2 disease, which remains one of the most challenging issues in thoracic oncology and thoracic surgery [23].
Nonetheless, the International Association for the Study of Lung Cancer (IASLC) proposed further subdivisions of pN1 and pN2 group patients who have a heterogenous prognosis, which was made by considering the number of LN metastasis and its location.
In response to these data, the IASLC recommended new descriptors for pN1 and pN2 patients by considering the location and the number of LN metastasis with the presence or absence of skip metastasis [24].
According to the IASLC, a significant difference was found between patients with single and multiple stations involvement in pN1 (pN1a versus pN1b) or pN2 (pN2a versus pN2b). There was also a difference in subdivided pN2a patients depending on whether skip metastasis was present or not (pN2a1 versus pN2a2), whereas no significant difference was shown between multiple N1 stations (N1b) and single N2 skip metastasis (N2a1, no N1 involvement) [24].
Up till now there is a controversy regarding either doing sampling or complete mediastinal lymph node dissection. However, it is generally agreed worldwide about the significance of complete lymph node dissection as a predictor for long term survival and hence it is recommended by many surgeons because it increases the accuracy of pathological staging [25].
We performed complete mediastinal lymph nodes dissection in our study. Dissecting a sufficient number of lymph nodes in both groups as recommended by the guidelines [23].
Regarding early postoperative complications encountered in both groups, we documented the following complications, prolonged air leak, anaemia, and atrial fibrillation. No other known complications were observed.
However, Kim et al. [17] documented early postoperative complications for the same procedures such as pneumonia, ARDS, chylothorax and vocal cord palsy. These complications we did not encounter during our study.
Atrial fibrillation and prolonged air leak were common complications between our study and that of Kim et al. [17].
The prolonged single lung ventilation time is related to the increased incidence of postoperative complications following lung resection. However, the addition of VAMLA will save the single lung ventilation time and hence decrease the incidence of postoperative complications [26].
Our percentage of conversion from VATS lobectomy procedure to open surgery was (31.3-33.3%). While Witte et al. [12] showed a lower conversion percentage in both groups (5-7%). Our causes of conversion were either from direct trauma to the pulmonary arterial vasculature during dissection or from indirect trauma to small pulmonary artery branches from traction injury during the manipulation of lung parenchyma.
R0 resection was achieved in 14 patients (87.5%) for group A. For group B, it was achieved in all the 6 patients (100%).
In our study, we showed that VAMLA is a clinically feasible procedure that can be done safety. In addition, VAMLA followed by VATS lobectomy allowed a removal of more mediastinal lymph nodes than VATS approach alone, suggesting that VAMLA can be a good complement to minimally invasive pulmonary resection for complete mediastinal lymphadenectomy in the surgical management for lung cancer. Similar findings were documented by Kim et al. [17].
Regarding the limitations of this study, it is non-randomized, prospective with observational data. So, our results may have been affected by unmeasured confounders.
Because all surgeries were performed by a single surgeon, the operative outcomes may have been influenced by the so called ‘surgeon bias’.
On the other hand, further researches are needed to be done to know whether extensive mediastinal lymph node dissection with VAMLA in addition to VATS lobectomy can be translated into the improved long-term survival and the reduced tumour recurrence in lung cancer patients [25].
Finally, it must be highlighted that the use of VAMLA followed by minimally invasive pulmonary resection in the surgical treatment of lung cancer is a relatively new area in medicine with a relatively small volume of data in medical literature available for discussing this topic.
Citation: Abdellatif AA, Allam A, Keshk S, Ramadan A, Abuarab W, Marasco RD, et al. (2020) Video-Assisted Thoracoscopic Lung Resection Following Video-Assisted Mediastinoscopic Lymphadenectomy In The Cure Of Non-Small Cell Lung Cancer. J Clin Trials 10: 416. doi: 10.35248/2167-0870.20.10.416
Received: 16-May-2020 Accepted: 29-May-2020 Published: 05-Jun-2020 , DOI: 10.35248/2167-0870.20.10.416
Copyright: © 2020 Abdellatif AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.