Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Review - (2019)Volume 8, Issue 4
Objectives: To evaluate the relation between baseline Vitamin D (VD) serum levels and incidence and severity of Pregnancy-Associated Morbidities (PAM).
Patients and methods: The study included 386 pregnant women attended at the 6th gestational week and after full clinical and obstetric examination, gave fasting blood samples for estimation of fasting blood glucose (FBG) and ELISA estimation of serum VD, insulin and interleukin-6 (IL-6). Insulin resistance (IR) was determined according to homeostasis model assessment IR (HOMA-IR) score that was considered abnormal at score of ≥ 2. Enrolled women were categorized according to a booking VD level as sufficient (Group A), insufficient (Group B) and deficient (Group C). Women of group C received daily oral VD (1000 IU) supplemental therapy (VD-ST) till delivery. Study outcomes included incidence of PAM and its relation to serum VD levels, and the effect of VD-ST on incidence and severity of PAM.
Results: Groups A-C included 86, 130 and 170 women, respectively and 263 women developed 424 PAM. PAM incidence and serum IL-6 levels were significantly higher in group B than other groups. PAM incidence and serum IL-6 levels showed negative significant correlations with serum VD levels. Moreover, PAM incidence showed positive significant correlation with serum IL-6 level. ROC curve analysis defined at booking serum VD as significant specific and serum IL-6 as significant sensitive predictors for PAM development. VD-ST allowed significant reduction of PAM with significant reduction of serum IL-6 and FBG levels and IR. Incidence of PAM was negatively correlated with receiving VD-ST that was found to be significant predictor for decreased PAM incidence.
Conclusion: Hypovitaminosis D and high IL-6 serum levels may underlie PAM development. VD-ST improved pregnancy outcome in women with VD deficiency to a level better than in women with insufficient VD levels; thus it is recommended as empirical therapy for pregnant women.
Hypovitaminosis D; Interleukin-6; Pregnancy-associated morbidities; VD-supplemental therapy
Vitamin D is a prohormon with multiple functions that extend beyond the regulation of the intestinal calcium absorption [1]. After being absorbed VD is bound to VD-binding protein and carried in blood stream to liver to undergo its first hydroxylation into 25-hydroxy VD [2]. Hydroxylated VD will be further hydroxylated in kidneys to 1, 25-dihydroxy VD; which is the biologically active form of VD [3] through binding to its receptors present in skeletal and non-skeletal tissues [4]. The placenta is an important source of VD and regulates its metabolism, and through the VD receptor in placental tissues, VD can regulate trophoblast function [5].
Pregnant women form one of the high risk groups facing hypovitaminosis D (HVD) [6], because the pregnant woman is the only source of VD for the fetus and the main sources of VD for pregnant women are sunlight, fortified dairy products, oily fish and dietary supplements [7]. Thus, maternal nutrition may influence both the overall pregnancy outcome, immune system of the mother and fetus and the growth trajectory of the fetus and infant [8].
VD deficiency/insufficiency is prevalent among pregnant women [9] and epidemiological studies had linked VD deficiency to adverse maternal and neonatal outcomes [5]. Thus, screening for HVD in the first trimester of pregnancy is important to determine the necessary preventive action [6].
Supplemental therapy (ST) during pregnancy is still a matter of discrepancy [10]; concerning VD-ST, the current evidence base could not allow definite conclusions regarding the optimal maternal circulating concentration of 25OH-VD during pregnancy, and how this might best be achieved [11]. VD-ST during pregnancy was associated with increased its circulating levels, birth weight, and length, but was not associated with other outcomes [12].
Hypothesis
Hypovitaminosis D (HVD) is a global problem and mostly has an impact during pregnancy on both the mother and fetus. HVD may predispose or aggravate Pregnancy-Associated Morbidities (PAM)
Objectives
The study aimed to evaluate the relation between baseline VD serum levels and frequency and severity of PAM
Design
Prospective selective therapeutic trial.
Setting
Zagazig University Hospital, Zagazig, Egypt.
The study protocol was approved by the Institutional Review Board (IRB) of Faculty of Medicine Zagazig University and all enrolled women signed a written fully-informed consent to participate in the study. To exclude the seasonal variations of VD levels, the study intended to collect all women attended the antenatal care unit at Zagazig University Hospital at the 6th week gestational age (6-GW) since March 21st till June 21st each year since March 2014 were eligible for clinical evaluation and after preliminary evaluation were asked to re-attend the clinic fasting on the next day to give blood samples for investigations.
Preliminary examination included full clinical and obstetric history taking, determination of women’s baseline anthropometric data and clinical data. Body mass index (BMI) was calculated according to the equation; BMI=weight (kg)/height (m2) and patients were classified as underweight: BMI<18.5 kg/m2, normal weight: BMI=18.5-24.9 kg/m2, overweight: BMI=25-29.9 kg/m2 and obese: BMI ≥ 30 kg/m2 [13].
Exclusion criteria included presence of multiple pregnancy, fetal congenital anomalies, current DM or essential hypertension, BMI >35 kg/m2, obesity-inducing endocrinopathy, evident manifestations of hypo-parathyroidism, thyrotocixosis, renal or hepatic diseases and women gave birth outside the hospital were also excluded from the study.
This study was conducted in compliance with all the applicable institutional ethical guidelines for the care, welfare and use of humans.
Blood sampling and investigations
Fasting blood samples were obtained from all women under complete aseptic conditions; blood samples obtained from the antecubital and were divided into three parts:
1. A part of blood sample was collected in a plane tube, till be clotted and then was centrifuged at 3000 rpm for 10 min. Serum was removed and collected in pyrogen-free Eppendorf tubes to be stored at -70°C until ELISA assayed for serum levels of 25OH-VD, using an ELISA kit from Cayman Chemical, Ann Arbor, MI, USA [4], insulin using ELISA kit from Quantikine R&D systems Inc. Minneapolis MN USA [14] and IL-6 using ELISA kit from Pelikine™ Inc., Concord, USA [15].
2. Another part was put in a tube containing sodium fluoride (2 mg sodium fluoride/ml blood) to prevent glycolysis. Plasma was separated by centrifugation and used for estimation of Fasting Blood Glucose (FBG) by glucose oxidase method [16].
3. A 3rd part was used for routine laboratory investigations according to hospital protocol including complete blood count, serum urea and creatinine and serum liver function tests (results are not included).
Evaluated parameters
1. Vitamin D sufficiency status was defined, at booking time, according to 25-OHD concentration as follows: ≥ 75 nmol/L sufficient level, 50-75 nmol/L insufficient level and <50 nmol/L deficient level [17].
2. Insulin resistance (IR) was measured by homeostasis model assessment IR (HOMA-IR) score. The HOMA-IR score was calculated as fasting serum insulin (μU/ml) x [FBG (mg/ ml)/18])/22.5 [18] was considered abnormal at HOMA-IR score of ≥ 2 [19]. IR parameters were estimated at both at booking time and the 36th GW
3. Gestational Diabetes Mellitus (GDM) was assesses at 36th GW using the 75g Oral Glucose Tolerance Test and diagnosed according to guides of the International Association of Diabetes and Pregnancy Groups for abnormal OGTT, as follows: FBG ≥ 92 mg/dl, 1-h BG ≥ 180 mg/dl and 2-h BG ≥ 153 mg/dl [20].
4. Pre-eclampsia (PE) was diagnosed by development of gestational hypertension after the 12th week GA in women who were normotensive at booking time with SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg on at least two occasions, 4 hours apart, and proteinuria defined as one dipstick measurement ≥ 2+ on a voided random urine sample [21].
5. Gestational Anemia (GA) was defined as Hb conc. of <11 gm/ dl either at booking time or at the 36th GW [22]. Hb deficit was calculated as the percentage of decrease in Hb conc. at the 36th GW in relation to that estimated at booking time.
Patients’ grouping
Patients were categorized according to estimated baseline serum vitamin D into three groups:
1. Patients with sufficient VD level were included as negative control group (Group A).
2. Patients with insufficient VD level were included as positive control group (Group B), were followed up and received no VD supplemental therapy (VD-ST).
3. Patients with deficient VD level were included as study group (Group C) and received VD-ST according to Grant et al. [23] as a daily oral dose of 1000 IU softgels to be taken with mail (Sunvite High Potency Vitamin D3 1000 IU; Puritan’s Pride, Inc., Oakdale, NY, USA). VD-ST was started since study enrollment, after giving blood sample, till delivery.
Study outcomes
• Primary outcome: the frequency of VD deficiency among studied women.
• Secondary outcomes included.
1. Frequency of pregnancy-associated morbidities (PAM) among studied groups and its relation with estimated serum VD levels.
2. The effect of VD-ST on frequency and severity of PAM.
Statistical analysis
Obtained data were presented as mean ± SD, numbers and percentages. Results were analyzed using One-way ANOVA test, paired t-test and Chi-square test (X2 test). Possible relationships were investigated using Pearson’s linear regression. Sensitivity & specificity of estimated parameters as predictors were evaluated using the Receiver Operating Characteristic (ROC) curve analysis judged by the Area under the Curve (AUC) compared versus the null hypothesis that AUC=0.05. Statistical analysis was conducted using the IBM SPSS (Version 23, 2015) for Windows statistical package. P value <0.05 was considered statistically significant.
The study included 450 pregnant women; 39 patients were excluded and 411 women were evaluated, 25 women had escaped during followup and 386 women were enrolled in the study. At booking time, estimated serum VD defined 86 women having sufficient VD level (Group A), 130 women had insufficient serum VD (Group B) and 170 women had deficient serum VD (Group C) (Figure 1). Apart from serum VD, other enrolment data of studied women showed nonsignificant (p>0.05) difference as shown in Table 1.
| Variable | Group A | Group B | Group C | P value | ||
|---|---|---|---|---|---|---|
| Number (%) | 86 (22.3%) | 130 (33.7%) | 170 (44%) | 0 | ||
| Age (years) | 27.3 ± 4.7 | 28.1 ± 4.3 | 27.4 ± 3.9 | 0.224 | ||
| Weight (kg) | 80.5 ± 8.5 | 77.3 ± 10 | 78.8 ± 9.4 | 0.054 | ||
| Height (cm) | 170.4 ± 3.7 | 169.2 ± 3 | 169.9 ± 3.5 | 0.148 | ||
| BMI (kg/m2) | 27.7 ± 3.1 | 27.2 ± 3.6 | 27.3 ± 3.2 | 0.089 | ||
| Gravidity | 2.4 ± 1.1 | 2.2 ± 0.8 | 2.2 ± 0.7 | 0.258 | ||
| Parity | 1.3 ± 1 | 1.1 ± 0.9 | 1.3 ± 0.8 | 0.346 | ||
| Systolic blood pressure (mmHg) | 118.5 ± 6.1 | 120.1 ± 8.3 | 120.8 ± 7 | 0.693 | ||
| Diastolic blood pressure (mmHg) | 72 ± 3 | 73.4 ± 5.9 | 75.4 ± 7.8 | 0.476 | ||
| Lab data | Serum VD (nmol/L) | 77.7 ± 2.1 | 58.6 ± 5.2 | 31.7 ± 9.3 | 0.0001 | |
| Hb conc. (gm) | 11.7 ± 0.8 | 11.6 ± 0.8 | 11.7 ± 0.9 | 0.686 | ||
| IR data | FBG (mg/dl) | 116.8 ± 7.7 | 116 ± 5.9 | 114.8 ± 7.1 | 0.079 | |
| FSI (µU/ml) | 5 ± 1.23 | 5.26 ± 1.23 | 5.22 ± 1.1 | 0.242 | ||
| HOMA score | 1.44 ± 0.35 | 1.5 ± 0.35 | 1.48 ± 0.31 | 0.396 | ||
Table 1: At booking data of women of studied groups
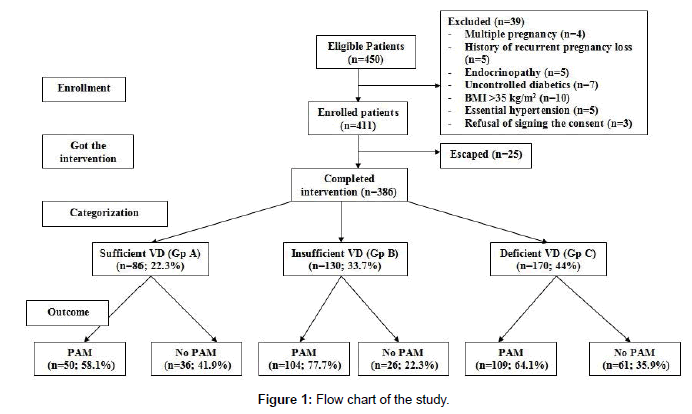
Figure 1: Flow chart of the study.
Throughout observation period 263 women developed 424 PAM for a frequency of 1.61 PAM/woman. Regarding the frequency among studied groups; 50 (58.1%), 104 (77.7%) and 109 (64.1%) women developed PAM in groups A-C, respectively (Figure 1). There was significant variance (p=0.001) among studied groups regarding the incidence of PAM among studied groups, but in favor of group A. The incidence of PAM among women of group B was significantly (p=0.0005) higher than groups A and C (p=0.0005 and 0.0027, respectively), but the difference was non-significantly (p=0.352) higher in group C than in group A.
Hemoglobin conc. determined at the 36th GW and calculated deficit than at booking concentration showed significant (p=0.0001 and 0.0089, respectively) difference between studied groups, but in favor of Group A. Development of Gestational Anemia (GA) was the most frequently recorded PAM; where 194 women developed GA. Differentially, 39, 75 and 80 women in groups A-C, respectively developed GA with non-significant (p=0.111) difference between the studied groups, but also in favor of group A (Table 2).
| Variable | Group A | Group B | Group C | P value | ||
|---|---|---|---|---|---|---|
| Hb. data | Conc. (gm) | 11 ± 0.77 | 10.64 ± 0.72 | 10.68 ± 0.72 | 0.0001 | |
| Deficit | 5.95 ± 1.5 | 8.53 ± 3.18 | 8.72 ± 3.72 | 0.0089 | ||
| GA | 39 (45.3%) | 75 (57.7%) | 80 (47.1%) | 0.111 | ||
| IR data | FBG | 122.8 ± 15.9 | 130.1 ± 18.6* | 123.9 ± 17.9 | 0.0029 | |
| FSI | 4.9 ± 1.2 | 5.4 ± 1.2 | 5.59 ± 1 | 0.00006 | ||
| HOMA score | <2 | 74 (83.7%) | 79 (60.8%) | 123 (72.4%) | 0.012 | |
| ≥2 | 14 (16.3%) | 51 (39.2%) | 47 (27.6%) | |||
| Mean | 1.5 ± 0.5 | 1.77 ± 0.54 | 1.72 ± 0.44 | 0.0009 | ||
| IL-6 (ng/ml) | 35.2 ± 16.4 | 44.4 ± 16 | 41.8 ± 14.4 | 0.0001 | ||
Table 2: At 36th GW laboratory findings and IR data of women of studied groups.
At booking time, no patient had IR, while at the 36th GW, 112 patients developed IR (29%); 14 in group A and 51 and 47 in groups B and C, respectively. The frequency of women developed IR at the 36th GW showed significant (p=0.012) variance among studied women with significantly higher frequency among women of group B compared to groups A (p=0.0003) and C (p=0.034) and non-significantly (p=0.063) higher IR women in group C versus group A. Among women developed IR, 59 women (16%) progressed to GDM, despite of the significantly higher frequency of IR in women with HVD (groups B and C), the frequency of women developed GDM was non-significantly higher among women had HVD compared to those had sufficient VD levels (Table 2).
Interestingly, all women showed significantly higher serum IL-6 compared to the standard control levels, as documented by manufacturer, with significant (p=0.0001) variance among studied groups. Moreover, women of group B had significantly higher serum IL-6 compared to those of groups A and C (p=0.00006 & 0.0012, respectively) with non-significantly (p=0.133) higher levels in women of group C than women of group A (Table 2 and Figure 2).
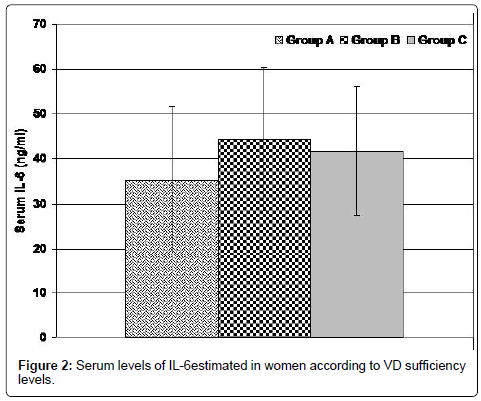
Figure 2: Serum levels of IL-6estimated in women according to VD sufficiency levels.
Thirty-four women (8.8%) developed pre-eclampsia; 5, 14 and 15 women in groups A-C, respectively with non-significant (p=0.453) variance among women in the three groups, but in favor of group A. Twenty-four women had preterm labor with non-significant (p=0.449) difference between the three groups, but in favor of group A.
Total incidence of PAM, frequency of women developed PE, serum IL-6 levels, frequency of women had preterm labor and HOMA-IR score showed negative significant correlation with at booking serum VD, in decreasing order of frequency, while Hb Conc. at 36th GW showed positive significant correlation with at booking serum VD. On the other hand, total incidence of PAM, HOMA-IR, GDM, PE and preterm labor showed positive significant correlation with at 36th GW serum IL-6 level, while at 36th GW Hb. Conc. was negatively correlated with serum IL-6 (Table 3).
| Variables | Serum VD | Serum IL-6 | ||
|---|---|---|---|---|
| r | p | r | p | |
| Serum IL-6 | -0.133 | 0.009 | - | - |
| Total frequency of PAM | -0.162 | 0.001 | 0.327 | 0.0003 |
| At 36th GW Hb Concentration | 0.133 | 0.009 | -0.183 | 0.0008 |
| At 36th GW HOMA-IR score | -0.122 | 0.017 | 0.27 | 0.0006 |
| GDM | -0.082 | 0.108 | 0.193 | 0.0009 |
| Frequency of PE | -0.14 | 0.006 | 0.134 | 0.008 |
| Frequency of preterm labour | -0.126 | 0.013 | 0.116 | 0.023 |
Table 3: Correlation between at booking serum VD and at 36th GW serum IL-6 with incidence rate of PAM.
ROC curve analysis defined at booking serum VD as significant (p=0.02) specific predictor with AUC=0.573 (±0.032; 95%CI=0.510- 0.636) and serum IL-6 as significant (p=0.0007) sensitive predictor with AUC=0.385 (±0.03; 95%CI=0.327-0.443) for development of PAM (Figure 3). Women of group C who received VD-ST had improved pregnancy outcome as manifested by the significantly lower incidence of PAM compared to women of group B. The reported beneficial effect of VD-ST was evidenced by the significant reduction of serum IL-6 (p=0.0012), FBG level (p=0.004) and IR (p=0.034). Moreover, the incidence of PAM was negatively (r=-0.142, p=0.014) correlated with receiving VD-ST and maintenance on VD-ST by women had deficient VD was found to be specific significant (p=0.010) predictor for decreased incidence of PAM with AUC=0.595 (±0.035; 95% CI=0.525- 0.664) (Figure 4).
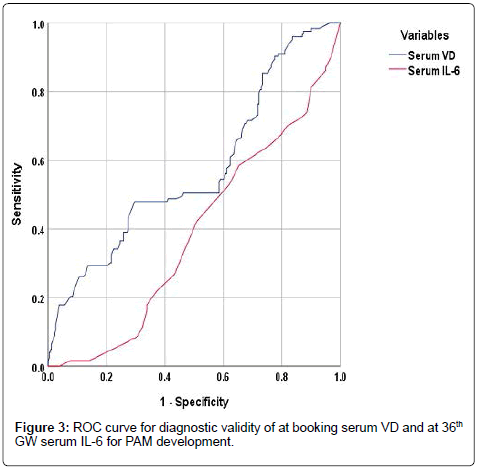
Figure 3: ROC curve for diagnostic validity of at booking serum VD and at 36th GW serum IL-6 for PAM development.
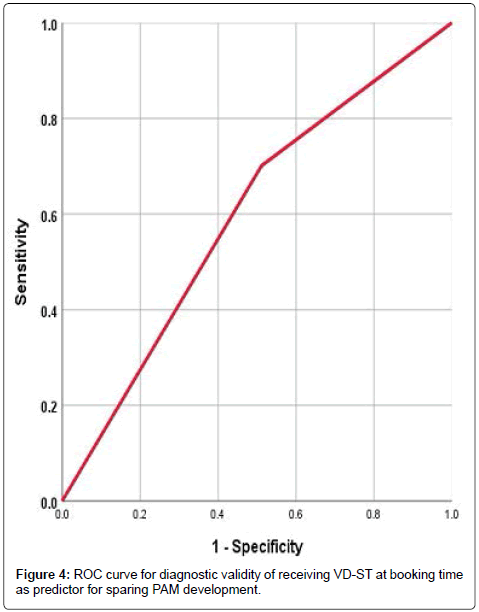
Figure 4: ROC curve for diagnostic validity of receiving VD-ST at booking time as predictor for sparing PAM development.
The reported incidence of HVD among studied pregnant women was 77.7%, considering timing of sample at the 6th GW, so HVD could not be attributed to pregnancy and was considered as pre-conception condition that most probably attributed to environmental and nutritional factors. In line with these data, Karras et al. [10] detected a prevalence of VD insufficiency and deficiency among pregnant women in Mediterranean region in ranges of 9.3-41.4% and 22.7-90.3%, respectively. Thereafter, Naseh et al. [9] reported VD deficiency in 27% and insufficiency in 73% of their sample of Iranian pregnant women. Moreover, Chakhtoura et al. [24] found the prevalence of HVD, defined as a 25 hydroxyvitamin D level <50 nmol/L, ranged between 44 and 96% in adults and 54-90% in pregnant women.
During the study duration, 263 women developed 424 PAM; 1.61 PAM/woman; differentially, at the 36th GW, 112 women developed IR and 59 of them progressed to GDM, 34 women developed PE and 24 women had preterm labor. There was significant variance among studied women categorized according to a booking serum VD levels regarding total and differential frequency of PAM.
Multiple clinical studies detected similar associations where Michalski et al. [25] found low VD status may be linked to reduce Hb concentration in pregnant women and Yuan et al. [26] reported that deficient maternal serum VD may be a risk factor for GA and it should be monitored especially for the high-risk pregnant women. Also, Amegah et al. [27] detected increased risk of preterm birth with serum VD levels <75 nmol/l with an inverse dose-response relation.
Recently, Windrim et al. [28] documented that VD deficiency during pregnancy has maternal and fetal implications, with increased risk of developing GDM, PE, preterm birth and small for gestational age birth weight. Also, Hong-Bi et al. [29] detected negative correlation between VD levels and adverse pregnancy outcomes. Moreover, Serrano et al. [30] reported increased probability of having PE among women with VD deficiency, relative to controls.
Serum IL-6 showed significant variance among studied groups with a positive significant correlation with incidence of PAM, HOMAIR, GDM, PE and preterm labor, while showed negative significant correlation with at 36th GW Hb. Conc. and deficit. These findings go in hand with Oztas et al. [31] who documented that higher 1st trimester IL-6 levels can independently predict adverse pregnancy outcomes. Also, Li et al. [32] suggested that high serum TNF-α and IL-6 levels are associated with pregnancy-induced hypertension and might be potential predictors for its prognosis. Moreover, Zhang et al. [33] found IL-6, IL-8, and IL-6/IL-10, serious inflammation and disordered lipid and glucose metabolisms were associated with GDM in women. Recently, Krasnyi et al. [34] documented that PE develops upon increased macrophage activity with subsequent increased serum levels of its factors; IL-10 and IL-6 leading to destruction of the placental trophoblastic cells.
Women with deficient serum VD who received VD-ST had improved outcome manifested as significantly lower incidence of PAM with significant reduction of serum IL-6 and FBG levels, and HOMA-IR score. VD-TS was found to specific significant (p=0.010) predictor for decreased incidence of PAM with AUC=0.595. In line with the beneficial effect of VD-ST on pregnancy outcome, Naseh et al. [9] suggested promoting consumption of VD-fortified foods and supplements among pregnant women to improve maternal and neonatal outcomes. Also, Krieger et al. [35] suggested that VD-ST during pregnancy should receive more attention in clinical practice as it was the most actionable determinant of VD status at the 3rd trimester and in neonatal cord blood and Wheeler et al. [36] recommended 1st trimester maternal VD screening and targeted supplementation for those “at risk”.
The dose of VD-ST was adjusted according to Grant et al. [23] in the form of 1000 IU softgels to be taken orally with mail starting since 1st antenatal visit till delivery. Similarly, Cooper et al. [37] found supplementation of women with cholecalciferol 1000 IU/day during pregnancy is safe and sufficient to ensure repletion of VD stores in pregnant women and Yilmaz et al. [38] documented that VD-ST with 1200 IU/day from the 12th GW can prevent neonatal VD deficiency. Also, Chakhtoura et al. [39] reported that in pregnant women VD dose of 1000-2000IU daily may be necessary to allow for the majority of this population to have serum VD level of 50 nmol/L.
Pregnancy is stressful period during women’s life and is associated with high incidence of morbidities. HVD and high IL-6 serum levels form a closed circuit that may underlie development of pregnancyassociated morbidities. VD-ST is a promising therapeutic option as it improved pregnancy outcome in women with VD deficiency to a level better than in women with insufficient VD levels; thus it is recommended to be applied in a similar dose as empirical therapy for all pregnant women.
Conflict of interest disclosed was none.
Both authors share in data collection, statistical analysis, paper design, writing and reviewing the paper before submission.
Citation: Helal KF, Harb OA (2019) Vitamin D Supplemental Therapy can Explore the Interplay Circuit between Hypovitaminosis D, High Serum IL-6 and Pregnancy- Associated Morbidities. J Women’s Health Care 8: 468. doi:10.35248/2167-0420.19.8.468
Received: 13-May-2019 Accepted: 28-May-2019 Published: 07-Jun-2019
Copyright: © 2019 Helal KF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.