Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
+44 1300 500008
ISSN: 1948-5964
+44 1300 500008
Research Article - (2021)Volume 13, Issue 4
Background: The clinical spectrum of COVID-19 disease includes asymptomatic infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure. The level of illness is associated with various individual factors. Therefore, this study aimed to assess factors that are associated with the development of symptoms among COVID-19 positive cases in a selected isolation and treatment center in Addis Ababa, Ethiopia.
Method: The study was conducted at Eka Kotebe General Hospital, COVID-19 Isolation and Treatment Center, Addis Ababa from May 11-24, 2020. All participants admitted to the center during the study period, 347 confirmed COVID-19 positive cases, were enrolled in the study. The dependent variable was having sign or symptom for COVID-19. Association of age, gender, Body Mass Index (BMI), blood type, comorbidities and history of travel with the presence of sign or symptoms was assessed. A logistic regression analysis was conducted to assess the associations after adjusting for selected covariates. Significant level for all variables was reported at 95% Confidence Interval.
Results: A total of 347 laboratory confirmed positive COVID-19 cases (mean age 33.9 ± 13.5) were included in the analysis. The large proportion (66%) of the study participants were males. Overall, 24% of the participants admitted to the hospital had at least one sign or symptom for COVID-19. Cough, headache, fever, sore throat and muscle ache were the most reported signs and symptoms. Cancer and HIV/AIDS were the leading comorbidities that the study participants reported. After adjusting for important covariates, gender, blood type, comorbidity and travel history were found to be significantly associated with having sign or symptom while being COVID-19 positive. However, age, BMI and income had no association with being symptomatic following the contraction of the COVID-19 infection.
Conclusion: Gender, blood group, comorbidities, travel history were found to be significantly associated with being symptomatic while having COVID-19 disease in Ethiopia. Age and BMI had no associations with developing COVID-19 sign or symptom. Closer monitoring and intensified prevention strategies to protect those who are highly likely to develop symptoms may help efficient use of scarce resources in the control of the pandemic. We recommend further study to elaborate on the cause of association and to advance the knowledge base available.
COVID-19; Pandemic; Ethiopia
ACT: Angiotensin Converting Enzyme; BMI: Body Mass Index; COVID: Corona Virus Disease; EPHI: Ethiopian Public Health Institute; rRT-PCRS: Reverse Transcription Real Time Polymerase Chain Reaction; SARS COV: Sever Acute Respiratory Syndrome Corona Virus
SARS-CoV-2, a novel coronavirus disease, was declared as a pandemic disease by the World Health Organization (WHO) on 7 April, 2020, it has now spread to more than 210 countries and territories [1]. The pandemic caused an unprecedented burden on health care systems and more specifically, on the public health emergency response systems of all countries in the world [2,3]. Fever, dry cough and fatigue were reported as the most common signs and symptoms of the disease. It also manifests as a systemic condition involving the respiratory system, (shortness of breath, sore throat, rhinorrhea, hemoptysis, and chest pain), gastrointestinal system (diarrhea, nausea, and vomiting), musculoskeletal system (muscle aches), and neurological system (headaches or confusion) [4,5]. The incubation period of the disease ranges from 1 to 14 days [3].
Studies from China published that the clinical spectrum of COVID-19 disease includes asymptomatic infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure [6,7]. Many participants of COVID-19 were hospitalized with pneumonia and these cases had significant association with admission to intensive care unit, death or mechanical ventilation [6,7].
A retrospective cohort study aimed to assess clinical course and risk factors for mortality of adult in participants with COVID-19 in Wuhan, China revealed that close to 50% of participants had comorbidities, the most common comorbidity was hypertension followed by diabetes and coronary heart disease [8]. Another study conducted to assess risk factors for COVID-19 clinical progression to monitor the stages of the disease in South Korea showed that there were significant differences in body temperature, initial chest x-ray findings and some signs and symptoms (chills, myalgia, and dyspnea) between COVID-19 participants with and without preexisting medical conditions. Hypertension, diabetes, and dyslipidemia were among those reported as preexisting medical conditions [9].
Increasing values of age, D-dimer, C-reactive protein, Sequential Organ Failure Assessment (SOFA) score, high body temperature, less albumin and history of diabetes were identified to be risk factors with the highest consistency as predictors for COVID-19 severity [1,8]. Being male and having blood type B and AB were also mentioned as being risk factors for severe forms of COVID-19 [9-11]. There is a clear and considerable dependency between age in disease acquisition (susceptibility) and outcome (fatality) [12,13]. A hospital-based study conducted among COVID-19 reverse transcriptase Real Time Polymerase Chain Reaction (rRT-PCR) positive cases in the USA showed that participants aged 65 years or over and with a BMI of 30 kg/m or higher were more likely to be hospitalized and in need of critical care [14]. However, in another literature, some findings indicate that abnormal chest x-rays, chills and diabetes influences the clinical progression and outcome of COVID-19 [15].
Assessment of risk factors for morbidity and mortality is important to guide prevention strategies as well as to target high-risk populations for enhanced care and support [14]. Furthermore, investigating factors associated with symptom development and aggravation of the disease from mild to severe is essential in providing efficient and appropriate management of participants with COVID-19 [15]. This study recruited participants with COVID-19 who were tested positive for coronavirus by rRT-PCR and admitted in the first case management center (Eka General Hospital, Isolation and Treatment Center) in Ethiopia. To the best of our knowledge, this is the first study conducted of Ethiopian positive COVID-19 cases to determine the factors associated with being symptomatic for the novel coronavirus disease.
Study design and settings
A hospital-based cross-sectional study of 347 positive COVID-19 cases was conducted at Eka Kotebe General Hospital, COVID-19 Isolation and Treatment Center. The Center is the first hospital designated to manage positive COVID-19 cases in Ethiopia. It has a capacity of admitting 600 cases. The study was conducted from 11 to 24 May 2020.
Study participants
The study participants consisted of adults who were positive by rRT-PCR test for COVID-19 and admitted to the Eka Kotebe General Hospital, COVID-19 Isolation and Treatment Center. The case management protocol developed by Ethiopian Ministry of Health, during the study period, guides admission of all laboratory confirmed cases, irrespective of clinical signs and symptoms.
Sampling
A census of 347 COVID-19 positive cases who were admitted to the Eka Kotebe General Hospital, COVID-19 Isolation and Treatment during the study period and who gave consent to participate in the study was included.
Definition of variables
Outcome variables: Symptomatic case was defined as any SARS- CoV-2 positive individual by rRT-PCR with at least one sign or symptom for COVID-19. The signs and symptoms include but not limited to cough, fever, headache, muscle pain and shortness of breath.
Non-symptomatic or asymptomatic was defined as any SARS-CoV-2 positive individual by rRT-PCR without any sign or symptom of COVID-19.
Explanatory variables: We used age, gender, comorbidity, blood type, monthly income, BMI and travel history as explanatory variables.
Imported cases are those who have history of travel from any country outside of Ethiopia in the past 15 days of their respective date of COVID-19 test positive.
Data collection
Treating physicians at the Eka Kotebe General Hospital, COVID-19 Isolation and Treatment Center collected the data from study participants. A two-day training on the data collection procedure and technique was provided. We used a structured data collection tool developed for the study. The data was collected using tablets and transferred to the Ethiopian Public Health Institute Server via REDCap system.
Data management and analysis
All data was cleaned and normality was assessed for the variables of interest. Frequency and distribution of both outcome and explanatory variables were analyzed followed by assessment of the association between variables. A logistic regression model was applied to examine the associations between having signs or symptoms and the explanatory variables (BMI, blood type, comorbidity, age, gender and travel history). We analyzed the crude and adjusted odds ratio with 95% confidence interval. The significance level was set at a P-value of less than 5%. Each model’s prediction value was 80% correct classification. All analyses were done using STATA 15 (Stata Corporation, College Station, Texas).
Descriptive, bivariate, and multivariate analyses to assess distribution and association of symptomatic cases with different variables were conducted.
Ethical clearance
Ethical approval from the Ethiopian Public Health Institute was secured (Ethics Ref. No. EPHI 6.13/690). The hospital management had assigned physicians to collect data from participants for the study. Before data collection, informed consent was obtained from each study participants. Data safety, security and confidentiality at all level were maintained.
Characteristics of the study participants
The study included a total of 347 participants admitted to Eka Kotebe General Hospital, COVID-19 Isolation and Treatment Center. Almost all study participants were under the age of 65 with a mean age of 34 (SD ± 14) years. The majority (66%) of them were male. On average, 53.6% of study participants had a monthly income less or equal to 3000 Ethiopian Birr (ETB) or 94 United States Dollars (Table 1).
| Characteristics | Number | Proportion (%) |
|---|---|---|
| Age | Mean ± SD 33.9 ± 13.5 |
Median ± IQR 29 ± 16 |
| Age>65 | 14 | 4.03 |
| Gender of the patient | ||
| Female | 119 | 34.3 |
| Male | 228 | 65.7 |
| Monthly income | ||
| ≤ 3000 birr (91USD) | 178 | 53.6 |
| >3000 birr (91USD) | 154 | 46.4 |
| Further case classification | ||
| Contact for acquisition unknown | 290 | 84.3 |
| Imported case or had contact with imported case | 54 | 15.7 |
Table 1: PCR parameters of the oligonucleotide primer sets used for the study.
A large proportion (42%) of the study participants were blood type O, followed by blood type A (27%). Around 66% of the participants had a BMI in a healthy weight range of 18.5-24.9 kg/m . Out of the total, 24% of the participants admitted to the hospital had at least one sign or symptom of COVID-19. Thirteen percent of the participants had also comorbidities and about 16% of the cases were either imported or had contact with imported cases (Table 2).
| Characteristics | Number | Percent (%) |
|---|---|---|
| Blood type | ||
| A | 65 | 26.6 |
| B | 51 | 20.9 |
| AB | 25 | 10.2 |
| O | 103 | 42.2 |
| BMI | ||
| <18.5 | 21 | 7 |
| 18.5-24.99 | 200 | 66.7 |
| 25-29.99 | 65 | 21.7 |
| >30 | 14 | 4.7 |
| Sign or symptom | ||
| Have at least one sign or symptom | 84 | 24.2 |
| Have no sign or symptom | 263 | 75.8 |
| Existing comorbidities | ||
| Don't have comorbidities | 301 | 86.7 |
| Have at least one comorbidity | 46 | 13.3 |
Table 2: Distribution of selected characteristics of the study participants.
Signs and symptoms description
Coughs, headaches, fever, sore throat and muscle ache were the most commonly reported signs and symptoms by COVID-19 participants (Table 3).
| Sign and symptoms | Frequency | Percentage |
|---|---|---|
| Cough | 48 | 57 |
| Headache | 47 | 56 |
| History of fever | 35 | 42 |
| Sore throat | 29 | 35 |
| Muscle aches | 24 | 29 |
| Fatigue | 24 | 29 |
| Chills | 20 | 24 |
| Joint ache | 20 | 24 |
| Loss of appetite | 19 | 23 |
| Runny nose | 11 | 13 |
| Shortness of breath | 9 | 11 |
| loss of taste or smell | 7 | 8 |
| Diarrhea | 6 | 7 |
| Nausea | 5 | 6 |
| Vomiting | 3 | 4 |
| Altered consciousness | 3 | 4 |
| Rash | 2 | 2 |
| Nosebleed, conjunctivitis and seizures each | 1 | 1 |
Table 3: Frequency of signs and symptoms exhibited among study participants.
Distribution of reported comorbidities among study participants
Participants’ history of preexisting medical conditions during admission for COVID-19 at the isolation and treatment center was assessed. Cancer was found to be the leading comorbidity reported by the study participants followed by HIV/AIDS. Hypertension, diabetes, and heart disease were also commonly reported. Chronic lung disease, chronic liver diseases and chronic neurological impairment were reported by very few participants. There was no patient with chronic hematological disorder and chronic kidney disease (Figure 1).
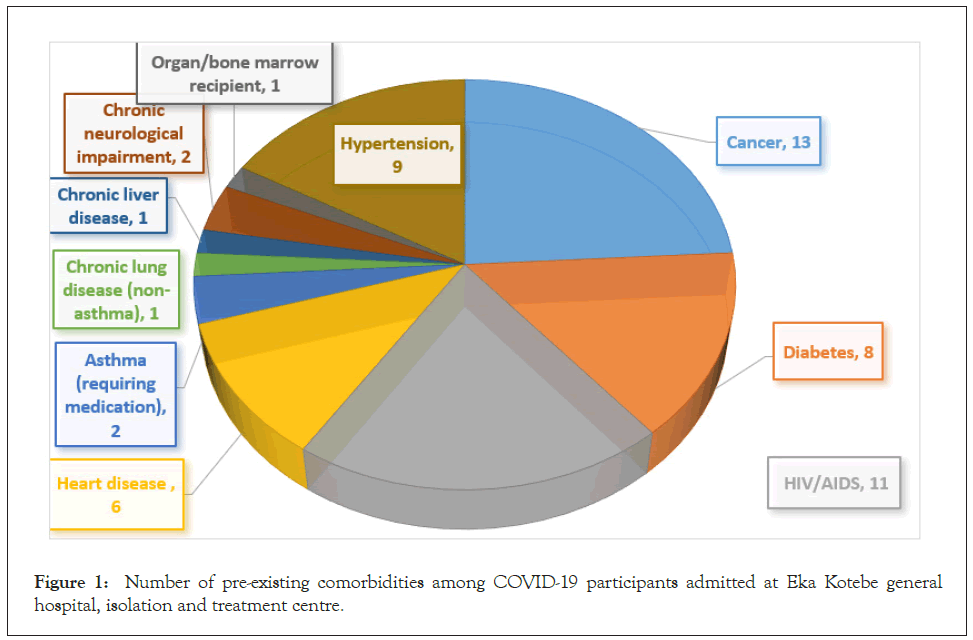
Figure 1: Number of pre-existing comorbidities among COVID-19 participants admitted at Eka Kotebe general hospital, isolation and treatment centre.
Factors associated with development of sign or symptoms for COVID-19
Association of age, gender, BMI, travel history, blood type and comorbidities with the presence of any signs or symptoms during hospital stay was assessed using a multivariable logistic regression. Gender, blood group, comorbidity and exposure for infection acquisition were found to be significantly associated with the development of symptoms (Table 4).
| Models | Predictors for COVID-19 symptom | Have symptoms number (%) | No symptom number (%) | AOR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Model 1 | Age in years | Median ± IQR | 30 ± 15 | 29 ± 16 | 0.994(0.962,1.028) | 0.745 |
| Model 2 | Gender | Female | 7(9.7) | 65(90.3) | 1 | |
| Male | 36(23.8) | 115(76.2) | 2.879(1.178,7.034) | 0.02 | ||
| Model 3 | Blood type | B | 15(29.4) | 36(70.6) | 1 | |
| A | 13(20) | 52(80) | 0.6(0.255,1.412) | 0.242 | ||
| AB | 5(20) | 20(80) | 0.6(0.190,1.895) | 0.384 | ||
| O | 15(14.6) | 88(85.4) | 0.41(0.181,0.923) | 0.031 | ||
| Model 4 | BMI | <18.5 | 4(19.1) | 17(80.9) | 1 | |
| 18.5-24.99 | 40(20) | 160(80) | 0.9(0.22,3.68) | 0.884 | ||
| 25-29.99 | 16(24.6) | 49(75.4) | 1.1(0.22,5.48) | 0.905 | ||
| ≥ 30 | 6(42.9) | 8(57.1) | 1.4(0.15,12.25) | 0.777 | ||
| Model 5 | Having any existing comorbidity | No comorbidity | 64(21.4) | 235(78.6) | 1 | |
| Has at least one comorbidity | 17(37.8) | 28(62.2) | 2.343(1.096,5.007) | 0.028 | ||
| Model 6 | Travel history | Contact not known | 32(16.7) | 160(83.3) | 1 | |
| Imported or have contact with imported case | 11(35.5) | 20(64.5) | 2.789(1.114,6.980) | 0.028 | ||
| Model 7 | Monthly income | ≤3000 birr ($ 91) | 38(21.4) | 140(78.6) | 1 | |
| >3000 birr ($ 91) | 44(28.6) | 110(71.4) | 1.5(0.89,2.43) | 0.129 | ||
Table 4: Determinants of being symptomatic for COVID-19 in Ethiopian, 2020.
The odds of sign or symptom development in males was almost three times (AOR=2.879; 95% CI: 1.178, 7.034; P=0.02) that of the odds in females. Having blood type O was also found to be inversely associated with the development of COVID-19 related signs and symptoms (P=0.031). Individuals with blood type O had 59% less likely to develop signs and symptoms compared to whose with blood type B (OR=0.41; 95% CI: 0.181, 0.923).
After adjusting for age, gender and history of travel, the probability of developing signs and symptoms among individuals with comorbidities was 2.43 (AOR=2.43; 95% CI: 1.06, 5.57). The odds of being symptomatic among imported or those who acquired the infection after contact with imported cases was nearly three times (AOR=2.789, 95% CI: 1.11, 6.98) compared to those reporting unknown contact as source of infection.
Age, BMI and monthly income had no association with being symptomatic following COVID-19 infection
Unlike previous studies, we did not find differences in symptom development in positive COVID-19 participants by age. According to the 2019 revised World Prospect Population, the majority of the Ethiopian population was young and between 15-65 years of age. The average age was 25 years; and those 65 years or older accounted only 4% of the total population [16]. Symptom development and increased risk of severe illness from COVID-19 is higher in old age buckets [17,18]. Our data did not have a representative sample of the 65 and older age group of the population to adequately assess the association between age and symptom development.
The proportion of females who acquired COVID-19 among all cases in this study was significantly smaller (34%) than males. This may suggest lower susceptibility of females to contract COVID-19 infection comparing to males. A lower proportion of females acquiring the disease was reported in many countries including Nepal (12%), Maldives (16%), Bahrain (12%), Qatar (9%) and Uganda (14%). However, in the Netherlands and Belgium, the proportion of positive COVID-19 females account for 62% and 63% of the total COVID-19 cases, respectively. These figures demonstrates that there is no consistent data showing the difference in probability of acquiring a COVID-19 infection by gender [19].
Males and females differed in their response to the COVID-19 infection resulting in gender differences in symptom development and disease severity [20]. A number of studies have also published similar findings showing that COVID-19 disease severity and death affects males disproportionately [20-22].
The variation in the innate immune systems and the extent of Angiotensin Converting Enzyme (ACE) in the body were thought to explain the difference in response to COVID-19 infection between males and females as described below.
A woman’s immune system is stronger than that of a man’s resulting in women being at lower risk of acquiring infectious diseases and experience a less severe form of the diseases [23]. Different sex hormones in males and females influence the response to viral infections by binding to hormone receptors expressed on immune cells. Estrogen, a female gender hormone, has an immune- stimulating effect while androgens, the male gender hormone, has an immune-suppression effect [24]. In a study that analyzed seasonal flu vaccine reactions, it was found that higher testosterone level, another male gender hormone, was associated with lower immunological reaction to vaccination [25]. In the process of X-chromosome development during embryonic stage, females are believed to get extra microRNA that may play a role in providing stronger immunity [26]. Stronger immune responses in females are not always beneficial. It may make females susceptible to immunopathology such as autoimmunity compared to males [27].
Angiotensin Converting Enzyme 2 (ACE2) is an enzyme molecule found on the cell membrane that connects the outer side of a cell with the inside part. ACE2 is abundantly available on the kidney, lung, blood vessels and intestine cells [28]. SARS-COV-2uses this receptor to enter the host cells. Therefore, it plays an important role in the pathophysiology and pathogenesis of COVID-19 infection [29-32]. The amount of ACE2 influences spread and invasion of the SARS-CoV-2 to target cells [33]. Plasma concentration of this ACE2 is higher in males than in females creating more favorable conditions in males for the virus to infect more cells of many organs including lung cells [32].
Findings from early observational studies showed association of blood type with risk of contracting COVID-19 and disease severity. Pre-print articles revealed that blood type O was related with a lower risk of getting infection compared with other blood types; and blood type A was associated with a higher risk of contracting COVID-19 compared with other types [33]. A lower proportion of blood type O and a higher proportion of blood type A was also observed among COVID-19 confirmed cases compared to those not infected with the SARS-CoV-2 [34]. A recent peer reviewed article published on The New England Journal of Medicine presented that blood type A had a 45% increased risk of acquiring the coronavirus and developing respiratory failure compared to individuals with non-A blood types. Conversely, blood type O had a 35% lower risk of becoming seriously ill with COVID-19. The team identified regions of genome that were linked with immune response in the lungs and coding blood types, highlighting the connection of blood type and severity of respiratory symptoms [35]. However, there are still contradicting findings on the association between blood type and severity of respiratory illness due to COVID-19 [10].
The correlation of blood types and the pattern of blood clot was well documented in the emerging literatures. Studies show that a higher risk of thromboembolism existed among people with non-O blood type than those with O blood type [36,37]. On another hand, as high as 31% of severely ill COVID-19 participants admitted to ICUs developed blood clotting problems, thrombotic complication [38]. In our study, blood type O was found to have a protective role for symptom development compared to blood type B. This indicates that there might be some sort of link between blood type and COVID-19 disease severity.
In our study, BMI had no association with having symptoms of COVID-19. Similarly, analysis of retrospective data on COVID-19 participants in Singapore found that participants with BMI ≥ 25 and participants with BMI <25 had no significant difference in clinical outcomes [39]. In contrary, several studies showed that being overweight and obesity had been associated with increased severity and mortality in the pandemic of H1N1 influenza and other respiratory viruses [40-43]. Sattar et al. also suggested that obesity (BMI>30) affects COVID-19 clinical outcome negatively by reducing protective cardiorespiratory reserve and potentiating immune dysregulation [44]. A recent systematic review from two different studies also revealed that participants with a high BMI were more likely to develop severe form of COVID-19 infections [45]. In our study, the majority of participants (67%) fall in the healthy BMI range of 18.5-29.99. Only 4.7% of participants were considered obese (BMI>30). This less proportion of obese participants might have masked the association between BMI and symptom development. The distribution of BMI in this study was similar with the obese Ethiopian adult population proportion in 2016 (6%) [46]. Majority of the study participants were relatively young and hence assumed to be physically active, which may result in lower BMI [47].
Our results showed that there was an increased likelihood of being symptomatic in participants with comorbidities. The presence of any preexisting medical condition was considered as a comorbidity. A study conducted in South Korea showed that COVID-19 cases with initial abnormal chest x-ray findings, chills and diabetes were more likely to have aggravated COVID-19 symptoms from mild to severe [15]. The study used hospital death as an endpoint while controlling for demographic and clinical characteristics of the participants. A study conducted in China also supported this finding, it underlined those comorbidities were associated with an increased risk of in-hospital death among participants with COVID-19 [48]. Furthermore, a literature review had published the existence of association between comorbidity and poor clinical progression of COVID-19 positive cases [49,50].
The study found that the odds of being symptomatic were higher among imported cases and their primary contacts than those who had acquired the infection through local transmission. A retrospective cohort study had revealed that contacts exposed to index cases in their symptomatic period had a higher risk of acquiring the infection than those exposed to participants with critically severe symptoms (RR: 2.15 vs 1.6) [51]. Possible explanations could be the loosening of virulence of the coronavirus as it spreads and mutates along its transmission chain. However, there is a debate among researchers on the mutation of coronavirus along transmission chains [52].
As with any observational study, the results of this study could be subjected to unmeasured confounding. Although we adjusted for several confounders in the multivariate models, there is a possibility of residual confounding. The design used is not appropriate to establish causality.
We have enrolled all volunteer participants in the study for the given period, and an adequate sample size was collected to address the hypothesis we developed at the beginning. To our knowledge, assessment of the relationship between selected health related variables and being symptomatic is the first in its kind in Ethiopia. Hence, it provides strong evidence in supporting the response to the COVID-19 pandemic.
Gender, blood group, comorbidities, travel history were found to be significantly associated with being symptomatic while being positive for COVID-19 disease in Ethiopia. Age and BMI had no associations with sign or symptom development in positive COVID-19 cases. Closer monitoring and intensified prevention strategies to protect those who are highly likely to develop symptoms when COVID-19 positive may help gear scarce resources in the control of the pandemic. Further study to elaborate the cause of association is recommended to advance the knowledge base available.
The authors hereby declare they have no conflicts of interest.
The study was funded by the Ethiopian Public Health Institute and Primary Health Care. No funding was obtained for publication.
Data could be made available upon request to the corresponding author, Saro Abdella, helen_saro@yahoo.com.
SA, MT and AD developed the idea of this analysis. AH analyzed the data. SA, MT and AD drafted the paper. All authors contributed to the interpretation of results, revision of the manuscript, and approval of the final version of the paper.
Not applicable.
The authors would like to thank the Ethiopian Public Health Institute, Primary Health Care and Eka Kotebe General Hospital, Isolation and Treatment Center management and staff. We are also most grateful to the study participants for their kind cooperation to provide us their personal information.
Citation: Abdella S, Hussen A, Defar A, Feleke A, Ahmad M, Rafera H, et al. (2021) Who Are at Most Risk to Develop Symptoms after SARS-Cov-2 Infection? Early Study in A Controlled Setting. J Antivir Antiretrovir. 13:225.
Received: 20-May-2021 Accepted: 03-Jun-2021 Published: 10-Jun-2021 , DOI: 10.35248/1948-5964.21.13.225
Copyright: © 2021 Abdella S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.