Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2022)Volume 12, Issue 6
To investigate the effects of Xingnaojing (XNJ) on the impairments of neurological functions and intestinal injury in Intracerebral Hemorrhage (ICH) mice. In vivo, mouse ICH model was prepared with stereotaxic injection of collagenase into the right basal ganglia. ICH mice were treated with XNJ (2.5, 5.0, 10.0 mL/kg intra-venous.). Garcia test and brain edema were evaluated. The Lipopolysaccharide (LPS), TNF-α and IL-1β in both blood and brain were assayed using Enzyme-Linked Immunoassay (ELISA) kits. The intestinal injury was also observed. In vitro, the model of gut injury was established using lipopolysaccharide-challenged Colon Carcinoma-2 (Caco-2) cells. And intestinal injury was evaluated by assaying Transepithelial Electrical Resistance (TEER) and FITC-D4 flux. In vivo, ICH mice exhibited a decrease of Garcia test score and an increase in brain edema. ICH induced a damage of intestinal tissue and increases of LPS, TNF-α, IL-1β. XNJ significantly improved the parameters mentioned above. In vitro, LPS induced a decrease of TEER, an expression of occludin, and an increase of FITC-D4 flux. However, XNJ not only augmented the level of TEER, expression of occludin but also reduced FITC-D4 flux in LPS-challenged Caco-2 cells. This study demonstrated that XNJ improves neurological function in ICH mice, in which, attenuating intestinal injury and inhibiting LPS translocation and the inflammation may play an important role.
Xingnaojing; Intestinal barrier dysfunction; Intracerebral hemorrhage; LPS translocation; Inflammation
Intracerebral Hemorrhage (ICH) is a potential life-threatening disease with a high initial mortality rate, from which several survivors were permanently disabled [1]. ICH not only induces neurological impairment and brain edema but also damages the peripheral organs, such as gut [2]. ICH can induce intestinal injury [3]. The increased intestinal permeability allows LPS into the blood circulation, which triggers neuroinflammation and causes further damage of brain tissue [4].
Intestinal epithelial cells are a key component of the gut barrier, and they participate in regulating immune response by secreting a variety of inflammatory mediators [5]. Tight Junction (TJ) is composed of transmembrane proteins (example: claudins, junctional adhesion molecule-A, and occludin) as well as intracellular plaque protein (example: ZO-1 and cingulin) [6]. Caco-2 is widely used in the study of structural and functional characteristics of the human intestinal barrier in vitro. LPS is a main component of the cell wall of gram-negative bacilli [7,8].
Xingnaojing (XNJ) is an agent prepared from Angong Niuhuang pill which is a classical prescription of traditional Chinese medicine [9,10]. Clinical trials and pharmacological studies suggested that XNJ alleviates brain injury and exerts neuroprotective effects [11-13]. However, whether XNJ can alleviate brain injury and gut barrier impairment in ICH is still unknown.
In this study, we investigate whether XNJ can improve neurological function and attenuate intestinal injury in intracerebral hemorrhagic mice. Then, intestinal injury was further investigated using LPS-challenged Caco-2 cells.
Xingnaojing and escin
Xingnaojing (Batch number: 180914) was purchased from Jiminkexin Pharmaceutical Co. Ltd (Jiangsu, China). XNJ is extracted with a modern technology from the main composite of Angong Niuhuang Pill. The quality of XNJ is ensured using the fingerprint technology which is according to the Standards of China Food and Drug Administration for control (approval no: WS3-B-3353-98-2003). In brief, muscone should be ≥ 0.1 mg/mL and borneol should be 0.8-1.2 mg/mL in XNJ [9,10]. Escin (Batch number: 2020060013), a reference drug, was purchased from Luye Pharmaceutical Co. Ltd (Shandong, China).
Animals
Male CD-1 mice weighting 28-36 g were obtained from the Jinan Pengyue Experimental Animal Breeding Co. Ltd. (Shandong, China; license number SCXK (LU) 20190003). All animals were kept in a 12 hours light/dark cycle at the temperature of 22 ± 2°C and under humidity of 50% ± 10% with free access to food and water.
Preparation of ICH mouse model
The experimental mice were anesthetized with ketamine/xylazine (100 mg/kg ketamine hydrochloride and 10 mg/kg xylazine via intraperitoneal injection), and positioned in a stereotaxic head frame (Stoelting, United states). The mice head was swabbed with 75% alcohol. The head hairs were removed to expose the calvarium. With a cranial drill (Ruiward Life Technology Co. China), a 1.0-mm burr hole was drilled at 0.2 mm in the front to bregma and 2.2 mm to the right lateral of the midline. A microsyringe was inserted with 3.5 mm in depth. Collagenase (0.5 μL 0.15 U/μL; Sigma, United states) was injected into the right striatum at the rate of 0.11 μL/min. After injection, the needle remained in theposition for 5 minutes to prevent reflux and then it was gently removed. The animals in the sham group were injected with sterile Positive Behaviour Support (PBS).
Cell culturing
The Caco-2 cells were provided by Procell Life Science and Technology Co. Ltd (Wuhan, China). The Caco-2 cells were cultured at 37°C under 5% CO2 in the medium of MEM (HyClone, United states) with 20% FBS (Gibco, United states) and 1% penicillin/streptomycin liquid (Shandong sparkjade biotechnology Co. Ltd. Shandong, China).
Lipopolysaccharide-challenged Caco-2 cells
The Caco-2 cells were plated at the Transwell permeable supports (Coring, United states). The culture medium was refreshed every alternate day. The cells were cultured for 8 days in the medium. The cells with TEER value> 1000 Ω * cm2 were used in the further experiments. The Caco-2 cells were challenged with LPS for 24 hours in order to establish a gut barrier dysfunction model that mimics the intestinal injury in ICH.
Experimental designs
Experiments 1, 2, and 3 were conducted in ICH mouse. Experiments 4 and 5 were conducted in Caco-2 cells are:
Experiment 1: Male CD-1 mice (n=42) were randomly divided into 6 groups with 7 mice in each group: Sham, ICH, escin (0.45 mg/kg), and XNJ (2.5, 5 or 10 mL/kg) groups. Escin was employed as the reference drug. Escin and XNJ were intravenously administered at 30 min, 24 hours, or 48 hours after the preparation of the ICH mouse model. Neurological deficits were measured at 24, 48, and 72 hours after the ICH. Brain edema and intestinal histopathology were evaluated at 72 hours after ICH.
Experiment 2: Male CD-1 mice (n=24) were randomly divided into 4 groups with 6 mice in each group: Sham, ICH, escin (0.45 mg/kg), and XNJ (5 mL/kg). Escin and XNJ intravenously injected at 30 min and 24 h after ICH. Brain edema and intestinal histo-pathology were evaluated at 48 hours after ICH.
Experiment 3: MaleCD-1 mice (n=24) were randomly divided into 4 groups with 6 mice in each group: Sham, ICH, escin (0.45 mg/kg), and XNJ (5 mL/kg). Escin and XNJ were intravenously injected at 30 minutes, 24 hours, or 48 hours after the ICH. The levels of LPS, TNF-α, and IL-1β in the blood and brain were measured at 72 hours after ICH.
Experiment 4: Caco-2 cells were divided randomly into 5 groups: control, LPS, and XNJ (1.25, 2.5, and 5 μL/mL) groups. XNJ and LPS (100 μg/mL) was added to the cultured media. TEER, FITC-D4 flux, and the level of TNF-α were evaluated at 24 hours after LPS exposure. TEER and FITC-D4 flux were measured thrice.
Experiment 5: Caco-2 cells were randomly divided into 3 groups: control, LPS, XNJ (5 μL/mL) groups. The expression of occludin protein was assayed by western blotting. All experiments were performed thrice.
Evaluation of neurological deficits
The neurological score was assessed by a researcher who is blinded to the experimental design at 24, 48, and 72 hours after ICH using the modified Garcia test. The mice were graded with the final score ranging from 0 to 21. In brief, the scoring system consisted of 7 tests with the score of 0-3 (0=worst; 3=best) for each, including 3 tests of sensory function (i.e. side stroking, vibrissae touch, and lateral turning) and 4 tests of motor functions (i.e. spontaneous activity, limb symmetry, and walking and climbing).
Brain edema assay
The mice were euthanized with overdose of isoflurane. Their brains were divided into 5 portions: Ipsilateral Basal Ganglia (Ipsi-BG), Ipsilateral Cortex (Ipsi-CX), Contralateral Basal Ganglia (Cont-BG), Contralateral Cortex (Cont-CX), and cerebellum. The cerebellum was employed as an internal reference. The brain tissues were weighed to access the wet weight of the brain tissues. Then, the tissue samples were dried at 100°C for 24 hours to determine their dry weight. Brain water content (%)=[(wet weight-dry weight)/wet weight]*100%.
Intestinal histopathology observation
The jejunum tissues were collected at 48 and 72 hours after ICH and fixed in 4% paraformaldehyde, followed by embedding in paraffin. The sections were then sliced into 5-μm thick section and stained with HE method. Microscopy was used to obtain images and the villus height was measured using the Image J software.
TNF-α, IL-1β, and LPS measurement
The blood and brain samples were collected at 72 hours after ICH. The blood sample was centrifuged to collect the sera. The brain tissues were weighted and homogenized with PBS (1 mg brain tissue with 4 μL PBS). XNJ and LPS were added to the cultured media. The level of TNF-α was evaluated at 24 hours after LPS exposure. Then, the supernatants and cultured media were collected and the levels of TNF-α, IL-1β, and LPS were assayed with the ELISA Kits (TNF-α and IL-1β from Solarbio Company, China and LPS from Nanjing Jiancheng Bioengineering Institution, China).
Cell viability assay
The viability of Caco-2 cells was determined by the MTT assay. The Caco-2 cells were seeded in 96-well plates and cultured with different concentrations of XNJ for 24 hours. Then, 20 μL of MTT (5 mg/mL) was added into each well and the Caco-2 cells were further incubated for 4 h. The MTT medium was removed and DMSO (150 μL/well) was added into the wells. The Optical Density (OD) of the solution was measured at 570 nm with a microplate reader (Molecular devices, China). The data was expressed as percent of control.
Measurement of TEER and FITC-D4 flux
To assess the intestinal barrier integrity. The TEER was measured using a Millicell-ERS voltohmmeter (Millipore, United states). The cultures were allowed to equilibrate for 30 minutes. Electrical resistance was measured and recorded on 3 consecutive measurements. Then, the resistances of the monolayers were recorded with the results multiplied with that of the monolayer area (1.1 cm2) after deduction of the background resistance. The TEER was expressed as percent of the control. The permeability of the Caco-2 cells was evaluated in the flux of FITC-D4 (Sigma-Aldrich, United states). After XNJ treatment, the apical and basolateral chambers of the Caco-2 cells were washed with PBS. Then, 1 mg/mL of FITC-D4 was added into the apical chamber and incubated for 1 hour. The fluorescent intensity of FITC-D4 was determined with the microplate reader (Molecular Devices) at the excitation of 490 nm and emission of 520 nm. The FITC-D4 flux was expressed as a percent of control.
Western blotting
The RIPA buffer supplemented with 1% PMSF was used to extract the whole protein from the Caco-2 cells. The concentration of proteins was quantified with a Bicinchoninic Acid (BCA) protein assay Kit. Then, 25 μg of protein was electrophoresed in 8% Sodium Dodecyl Sulphate-Polyacrylamide (SDS-PAGE) gel electrophoresis and transferred into polyvinylidene fluoride membranes. The membranes were blocked with a blocking buffer for 10 minutes at the room temperature, and incubated with anti-occludin (1:5000) and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000) overnight at 4°C. Subsequently, the membranes were incubated with secondary antibodies (1:1500) for 1 hour at the room temperature. The proteins were quantified using an Enhanced Chemiluminescence Kit (ECL) and the Image J software.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) 22.0 software. All data were expressed as mean ± Standard Deviation (SD). The data were Analyzed with One-way Analysis of Variance (ANOVA), followed by Lysergic acid diethylamide (LSD) test for multiple comparisons. P< 0.05 was set to indicate statistically significant difference.
XNJ improved the neurobehavioral functions in ICH mice
The scores of garcia test significantly decreased in the ICH group at 24, 48, and 72 hours after ICH (P< 0.01) when compared with that of the sham group. XNJ at the dose of 2.5, 5, and 10 mL/kg significantly increased the score of Garcia test (P< 0.05 or P< 0.01) compared with that in the ICH group (Figures 1A-1C). These results indicated that XNJ improved the neurological functions after ICH (Figure 1A).
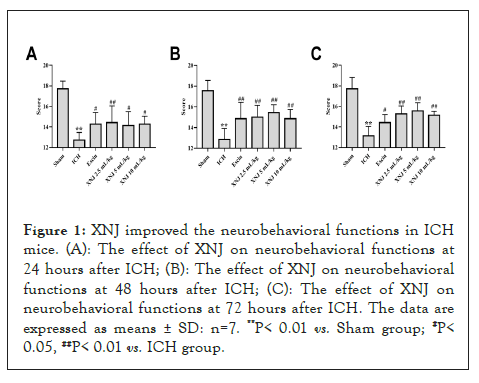
Figure 1: XNJ improved the neurobehavioral functions in ICH mice. (A): The effect of XNJ on neurobehavioral functions at 24 hours after ICH; (B): The effect of XNJ on neurobehavioral functions at 48 hours after ICH; (C): The effect of XNJ on neurobehavioral functions at 72 hours after ICH. The data are expressed as means ± SD: n=7. **P< 0.01 vs. Sham group; #P< 0.05, ##P< 0.01 vs. ICH group.
XNJ reduced brain edema in ICH mice
At 48 hours after ICH in Figure 2A, the brain water content in Ipsi-BG, Cont-BG, and Cont-CX were augmented (P< 0.01). Treatment with XNJ at the dose of 5 mL/kg reduced the brain edema of Ipsi-BG (P< 0.01). At 72 hours after ICH (Figure 2B), escin or XNJ reduced the brain edema of Ipsi-BG and Ipsi-CX (P< 0.05 or P< 0.01) (Figure 2A).
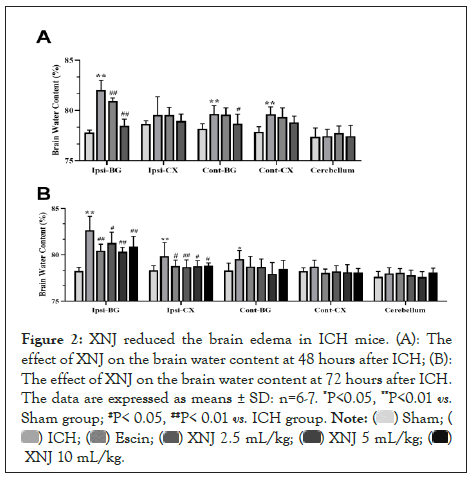
Figure 2: XNJ reduced the brain edema in ICH mice. (A): The effect of XNJ on the brain water content at 48 hours after ICH; (B): The effect of XNJ on the brain water content at 72 hours after ICH. The data are expressed as means ± SD: n=6-7. *P<0.05, **P<0.01 vs. Sham group; #P< 0.05, ##P< 0.01 vs. ICH group.
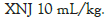
XNJ alleviated the pathological intestinal injury in ICH mice
At 48 hours of the ICH, there was an injury observed in jejunum, including intestinal epithelial cells necrosis, loss, and villi desquamation. XNJ at the dose of 5 mL/kg ameliorated the ICH-induced injury of the jejunum (Figures 3A and 3B). The villus height of the jejunum was shortened (P< 0.01) at 48 hours after ICH when compared with the sham mice. Escin or XNJ at the dose of 5 mL/kg increased (P< 0.01) the villus height of the jejunum (Figure 3C). At 72 h after the ICH, escin or XNJ at the dose of 2.5, 5 or 10 mL/kg ameliorated the ICH-induced injury of the jejunum (P< 0.01, Figure 3B) and increased the villus height of the jejunum (P< 0.01) (Figure 3A).
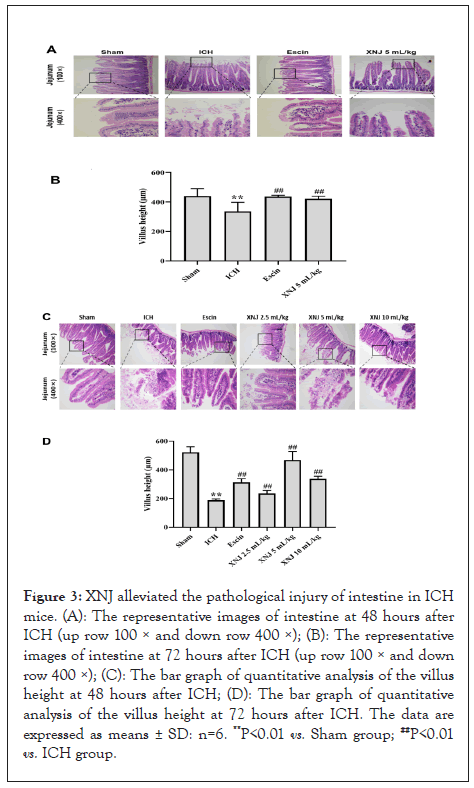
Figure 3: XNJ alleviated the pathological injury of intestine in ICH mice. (A): The representative images of intestine at 48 hours after ICH (up row 100 × and down row 400 ×); (B): The representative images of intestine at 72 hours after ICH (up row 100 × and down row 400 ×); (C): The bar graph of quantitative analysis of the villus height at 48 hours after ICH; (D): The bar graph of quantitative analysis of the villus height at 72 hours after ICH. The data are expressed as means ± SD: n=6. **P<0.01 vs. Sham group; ##P<0.01 vs. ICH group.
XNJ decreased the level of LPS in the blood and brain of ICH mice
LPS level in the blood in Figure 4A and brain Figure 4B was significantly increased (P< 0.01) at 72 hours after ICH. XNJ at the dose of 5 mL/kg markedly decreased (P< 0.01) the LPS level of the blood and brain (Figure 4A).
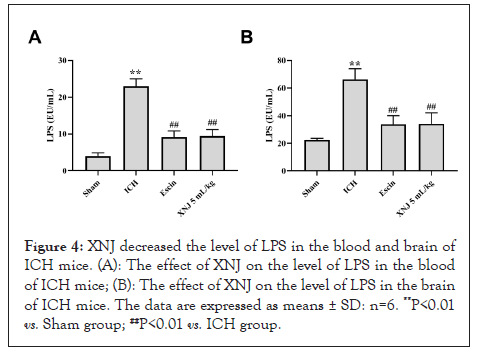
Figure 4: XNJ decreased the level of LPS in the blood and brain of ICH mice. (A): The effect of XNJ on the level of LPS in the blood of ICH mice; (B): The effect of XNJ on the level of LPS in the brain of ICH mice. The data are expressed as means ± SD: n=6. **P<0.01 vs. Sham group; ##P<0.01 vs. ICH group.
XNJ decreased the levels of TNF-α and IL-1β in the blood and brain of ICH mice
The levels of TNF-α and IL-1β in the blood and brain of the ICH group were increased (P< 0.01) when compared with those of the sham group. XNJ at the dose of 5 mL/kg decreased (P< 0.01) the levels of TNF-α and IL-1β in the blood and brain of ICH mice (Figures 5A-5D).
Figure 5: XNJ decreased the levels of TNF-α and IL-1β in the blood and brain of ICH mice. (A): The effect of XNJ on the level of TNF-α in the blood of ICH mice; (B): The effect of XNJ on the level of TNF-α in the brain of ICH mice; (C): The effect of XNJ on the level of IL-1β in the blood of ICH mice; (D): The effect of XNJ on the level of IL-1β in the brain of ICH mice. The data are expressed as means ± SD: n=6. **P<0.01 vs. Sham group; ##P<0.01 vs. ICH group.
XNJ alleviated the epithelial barrier dysfunction in Caco-2 cells monolayers
XNJ did not show any cytotoxic effect on Caco-2 cells at the concentration of 4 μL/mL (P> 0.05, Figure 6A). And the temporal TEER in Caco-2 cells (Figure 6B). Compared with the control group, LPS exposure induced a decrease of TEER in Caco-2 ells (P< 0.01, Figure 6C). XNJ (1.25, 2.5, and 5 μL/mL) augmented the TEER (P< 0.01, Figure 6C). When compared with the control group, LPS exposure increased the FITC-D4 flux (P<0.01, Figure 6D). XNJ (1.25, 2.5, and 5 μL/mL) significantly inhibited the LPS-induced FITC-D4 flux (P<0.01, Figure 6D). In the LPS-challenged Caco-2 cells, the expression of occludin was markedly decreased (P< 0.01). Treatment with XNJ augmented the expression of occludin (P< 0.01) (Figure 6D).
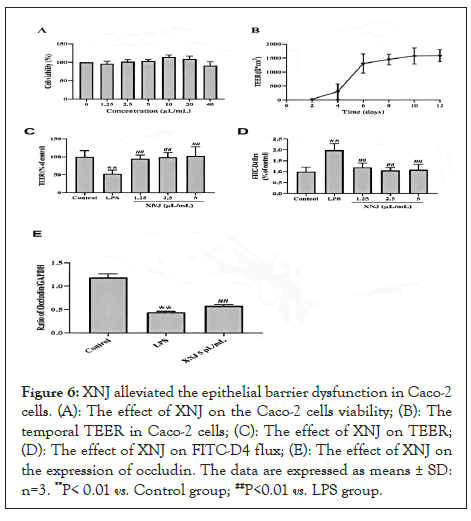
Figure 6: XNJ alleviated the epithelial barrier dysfunction in Caco-2 cells. (A): The effect of XNJ on the Caco-2 cells viability; (B): The temporal TEER in Caco-2 cells; (C): The effect of XNJ on TEER; (D): The effect of XNJ on FITC-D4 flux; (E): The effect of XNJ on the expression of occludin. The data are expressed as means ± SD: n=3. **P< 0.01 vs. Control group; ##P<0.01 vs. LPS group.
Effect of XNJ on the level of TNF-α in the culture medium
The level of TNF-α in the culture medium was evaluated. The exposure of LPS resulted in a significant increase of the TNF-α level (P< 0.01). While XNJ markedly reduced (P< 0.01) the level of TNF-α in the culture medium (Figure 7).
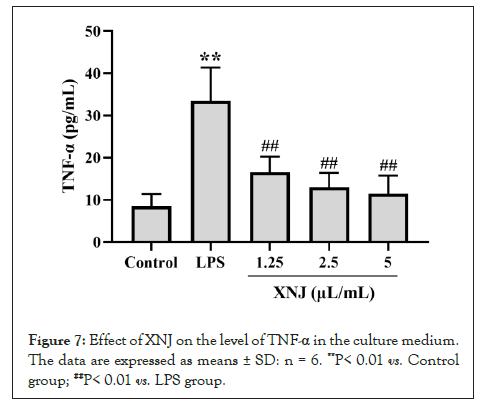
Figure 7: Effect of XNJ on the level of TNF-α in the culture medium. The data are expressed as means ± SD: n = 6. **P< 0.01 vs. Control group; ##P< 0.01 vs. LPS group.
ICH is associated with uncontrolled high blood pressure and antithrombotic or thrombolytic therapy. ICH, although less common, is significantly more lethal than ischemic stroke, with 30-day case-fatality rates that are two to six times higher [14]. ICH increases the intracranial pressures, leading to the destruction of blood brain barrier and brain edema. In the present study, ICH mice showed a decrease of garcia test sore and an increase of brain water extent, indicating a severe brain injury. These results were consistent with the previous reports. Based on the results that escin at dose of 0.45 mg/kg improved cognitive deficits and reduced brain edema after stroke [15,16]. Escin was employed as a reference drug in this experiment. The results showed that XNJ not only improved the neurological functions but also reduced the brain water content in ICH mice.
ICH not only leads to primary brain injury but also causes damages to the peripheral organs, such as gastrointestinal tract, lung, spleen, and heart [17]. Intestinal injury is one of the serious complications of ICH. ICH patients presenting with intestinal barrier dysfunction are observed with poor outcomes, such as increased mortality rates and severe impairment of the neurological functions. Both brain-gut axis and Hypothalamic Pituitary Adrenal (HPA) axis play critical roles in maintaining the integrity and permeability of intestinal barrier and preventing the translocation of bacteria and LPS in gut [18]. In the early stage of ICH, HPA axis and Sympathetic Nervous System (SNS) are activated. The activation of SNS and the inhibition of the Parasympathetic Nervous System (PNS) are associated with the intestinal injury after ICH [18]. The present study demonstrated that XNJ ameliorated ICH-induced damage of the jejunum. XNJ inhibited the exfoliation of intestinal cell and attenuated the damage of villi. These findings indicated that the XNJ-mediated improvement of neurological functions may be related to its protective effect on intestine in ICH mice.
To our knowledge, the mechanisms of action of XNJ reported by previous studies focused on elucidating the neuroprotective properties of XNJ. The role of XNJ in the gut is ignored. In order to know the mechanisms of action of XNJ, a series of in vitro experiments were performed. In clinic, the recommended dose of XNJ was 10 or 20 mL per time, once a day [13]. The blood volume of patient was 70 mL/kg [19]. Therefore, the concentration of XNJ in circulation will be 1.9 or 6.2 μL/mL. Based on the above calculation, the concentrations of XNJ (1.25, 2.5 or 5 μL/mL) were selected in the experiments in vitro.
TEER and FITC-D4 are widely used to evaluate the integrity and permeability of the intestinal barrier in Caco-2 cells [20]. In this study, the value of TEER was decreased and the FITC-D4 flux was increased in LPS-induced Caco-2 cells. However, treatment with XNJ improved these changes. TJ protein plays a key role in maintaining the intestinal barrier function. The expression of occludin was down regulated in Caco-2 cells after LPS exposure. However, XNJ upregulated the expression of occludin in Caco-2 cells and thus ameliorated the impairment of intestinal barrier function.
It is well-known that there is a large amount of LPS in intestine. LPS will cross the intestinal barrier and enter systemic circulation after ICH-induced intestinal injury. The level of LPS in blood is an indicator for evaluating the dysfunction of the intestinal barrier [21]. The LPS of blood also plays an important role in resulting in a systemic inflammatory response and exacerbate the destruction of the blood-brain barrier [22]. Furthermore, the LPS even cross blood-brain barrier and trigger a neuroinflammation, which plays a key role in the secondary brain injury of ICH [23]. This study demonstrated that XNJ inhibited LPS translocation and the production of TNF-α, IL-1β both in the blood and brain tissue in ICH mice. Additionally, XNJ also decreased the production of TNF-α in LPS-challenged Caco-2 cells.
To our knowledge, the mechanisms of action of XNJ reported by previous studies were associated mainly with elucidating the neuroprotective properties of XNJ. The possible role of XNJ played in the gut was ignored. In the current study, XNJ improved the ne1urological function and showed a protective effect on the gut barrier after ICH. XNJ maintained the integrity of intestine and the permeability of the gut barrier and therefore inhibited the inflammatory responses. These findings suggested that XNJ might attenuate ICH-induced damage of intestinal tissue and then ameliorated indirectly brain injury. Based on the results of the present study, it is reasonable to deduce that the mechanisms of action of XNJ to attenuate the ICH-induced brain injury is related to inhibit the systemic inflammation, especially, the neuroinflammation in brain, in turn, playing role of improving the neurological function. In conclusion, the present study demonstrated that XNJ not only alleviated brain injury but also attenuated intestinal injury. Based on the knowledge of the physiological and pathological processes of ICH-induced intestinal injury and the present findings, strategies attenuating the complication of intestinal injury following ICH will be a promising therapeutic approach in ICH patients.
• Ce Zhang and Yiqian Fan has acknowledged regarding conceptualization, methodology, investigation, writingoriginal draft.
• Yunqi Yang and Shumin Yuehas acknowledged regarding conceptualization, methodology and investigation.
• Min Li has acknowledged regarding conceptualization, methodology.
• Tian Wang and Fenghua Fu has acknowledged regarding methodology, writing-review and editing.
• Tian Wang and Fenghua Fu has acknowledged regarding resources, formal analysis.
This work was supported by the National Natural Science Foundation of China (Grant numbers 81873039)
None to report
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zhang C, Fan Y, Yue S, Yang Y, Li M, Wang T, et al. (2022) Xingnaojing: Improving Neurological Function and Attenuates Intestinal Injury in Intracerebral Hemorrhagic Mice. Trans Med. 12:274.
Received: 10-Nov-2022, Manuscript No. TMCR-22-20038; Editor assigned: 14-Nov-2022, Pre QC No. TMCR-22-20038 (PQ); Reviewed: 28-Nov-2022, QC No. TMCR-22-20038; Revised: 05-Dec-2022, Manuscript No. TMCR-22-20038 (R); Published: 12-Dec-2022 , DOI: 10.35248/2161-1025.22.12.274
Copyright: © 2022 Zhang C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.